我们决定向甘孜泸定、雅安石棉捐赠300万元现金、300万元物资,目前已成功对接甘孜州红十字会、雅安市红十字会,今天下午已经完成打款,物资根据当地所需正在紧急集结。对于灾区需要的其他支持,我们也当全力以赴。”
9月6日下午,四川科伦药业股份有限公司相关负责人告诉记者,针对四川泸定6.8级地震中受灾严重的泸定县和石棉县,他们紧急启动灾害应急处理方案,并进行现金和物资捐赠。
2026-01-16
CHENGDU, China, Jan. 16, 2026 /PRNewswire/ -- From January 12 to 15, 2026, the 44th J.P. Morgan Healthcare Conference (JPMHC) was held in San Francisco, California, USA. Dr. Ge Michael, President and CEO of Sichuan Kelun-Biotech Biopharmaceutical Co., Ltd. ("Kelun-Biotech" or the "Company", 6990.HK), was invited to attend the conference and delivered a keynote speech on the morning of January 15 (local time) and presented the Company's latest achievements in drug R&D, commercialization, and globalization, and outlined its innovation strategy and future development plans.
Since initiating its innovation journey in 2012, Kelun-Biotech has rapidly emerged as a leader in China's innovative drug field, building differentiated technology platforms and robust R&D pipelines. Leveraging its global leading OptiDC™ platform for antibody-drug conjugates (ADCs) and novel drug conjugates (DCs), the Company continuously advances the differentiated development of ADCs and novel DCs, forming a gradient portfolio for treating multiple tumor types. Currently, Kelun-Biotech has two ADC products on market: sacituzumab tirumotecan (sac-TMT, 佳泰莱®) and trastuzumab botidotin (舒泰莱®), covering breast cancer and lung cancer indications. Additionally, nine uniquely designed ADC and novel DC drugs—including cutting-edge directions such as bispecific ADC and radiopharmaceutical conjugate (RDC) are in clinical stage. For high-incidence tumor types in China, such as breast cancer, lung cancer, and gastrointestinal tumors, the Company has initiated nine pivotal studies, while multiple Phase II clinical studies targeting gynecological tumors are progressing steadily. Furthermore, Kelun-Biotech has also developed several non-DC candidates and expanded indications into non-oncology areas.
Over the past year, Kelun-Biotech has presented multiple research findings at international academic conferences and published in authoritative journals: three were selected for oral presentations at the American Society of Clinical Oncology (ASCO) annual meeting, and three were selected as Late-Breaking Abstracts (LBA) for oral presentations at the European Society for Medical Oncology (ESMO) Congress. Among these, the results of the OptiTROP-Lung04 study of sac-TMT for treating EGFR-mutant Non-Small Cell Lung Cancer (NSCLC) after TKI therapy were presented at the Presidential Symposium session of ESMO and simultaneously published in The New England Journal of Medicine, highlighting its global academic and clinical value.
In terms of commercialization, Kelun-Biotech has formed a competitive initial product portfolio. Its core product, the TROP2-directed ADC sac-TMT, has been approved in China for three indications: second-line and above triple-negative breast cancer, second-line and third-line EGFR-mutated NSCLC. The HER2-directed ADC trastuzumab botidotin was approved last year for second-line and above HER2-positive breast cancer, becoming the first domestically developed HER2-directed ADC approved for this indication. Furthermore, the anti-EGFR monoclonal antibody Cetuximab N01 (达泰莱®) for RAS wild-type colorectal cancer and the anti-PD-L1 monoclonal antibody tagitanlimab (科泰莱®) for nasopharyngeal carcinoma have been launched. Another small-molecule RET inhibitor A400 is expected to be approved within the year, bringing the total number of commercialized products in China to five. Currently, three of the Company's commercialized products, covering five indications, have been included in the National Reimbursement Drug List (NRDL), further benefiting a broad population of cancer patients.
While strengthening its presence in the domestic market, Kelun-Biotech is actively expanding overseas. It has established collaborations with MSD, Ellipses, Windward Bio and Crescent Biopharma to maximize the value of its pipeline value and corporate worth. Among these, MSD is evaluating 16 global Phase III clinical studies of sac-TMT.
The breakthroughs in both clinical development and commercialization are driven by the Company's sustained investment in innovative R&D. With over a decade of accumulation in the ADC field, Kelun-Biotech's proprietary OptiDC™ platform enables differentiated design of drug candidates, which combines specific targets or targeting mechanisms with the most suitable payload-linker strategy to balance efficacy and safety. Additionally, the company is adopting a "multi-pronged" innovation strategy to continuously enhance platform capabilities. By exploring novel targets, new payloads, and diverse conjugation technologies, it is expanding the application boundaries across both oncology and non-oncology fields.
Looking ahead, Kelun-Biotech will consolidate its foundations in R&D, technologies, platforms, and operations by Executing five key development strategies. Concurrently, the Company will elevate its globalization strategy, enhancing its capabilities in product development, registration, and commercialization in ex-China market, advancing on its path to becoming a world-class biopharmaceutical company.
Please refer to the "Investor Relations-Investor Calendar" page of the company's official website for the presentation materials. You can visit the website for further details.
About Kelun-Biotech
Kelun-Biotech (6990.HK) is a holding subsidiary of Kelun Pharmaceutical (002422.SZ), which focuses on the R&D, manufacturing, commercialization and global collaboration of innovative biological drugs and small molecule drugs. Kelun-Biotech focuses on major disease areas such as solid tumors, autoimmune, and metabolic diseases, and in establishing a globalized drug development and industrialization platform to address the unmet medical needs in China and the rest of world. Kelun-Biotech is committed to becoming a leading global enterprise in the field of innovative drugs. At present, Kelun-Biotech has more than 30 ongoing key innovative drug projects, of which 4 projects have been approved for marketing, 1 project is in the NDA stage and more than 10 projects are in the clinical stage. Kelun-Biotech has established one of the world's leading proprietary ADC and novel DC platforms, OptiDC™, and has 2 ADC projects approved for marketing, and multiple ADC and novel DC assets in clinical or preclinical research stage. For more information, please visit https://en.kelun-biotech.com/.
2026-01-05
CHENGDU, China, Jan. 5, 2026 /PRNewswire/ -- Sichuan Kelun-Biotech Biopharmaceutical Co., Ltd. ("Kelun-Biotech" or the "Company", 6990.HK) announced that its Investigational New Drug (IND) application for SKB105 (also known as CR-003), an internally developed integrin beta-6 (ITGB6)-targeted antibody-drug conjugate (ADC), has been approved by the Center for Drug Evaluation (CDE) of the National Medical Products Administration (NMPA) of China for the treatment of advanced solid tumors.
In December 2025, Kelun-Biotech and Crescent Biopharma, Inc. ("Crescent") entered into a strategic collaboration for SKB105/CR-003 and SKB118 (a PD-1 x VEGF bispecific antibody, also known as CR-001). Under the collaboration, Kelun-Biotech granted Crescent exclusive rights to research, develop, manufacture and commercialize SKB105/CR-003 in the United States, Europe and all other markets outside of Greater China. In addition, Crescent granted Kelun-Biotech exclusive rights to research, develop, manufacture and commercialize SKB118/CR-001 in Greater China. The IND application for SKB118/CR-001 has been cleared by the U.S. Food and Drug Administration (FDA) with a global Phase I/II clinical trial for the treatment of advanced solid tumors is set to commence shortly. Kelun-Biotech plans to submit an IND application for SKB118/CR-001 to the Center for Drug Evaluation of the National Medical Products Administration of China in the near future.
About SKB105 (also known as CR-003)
SKB105 is a differentiated ADC targeting ITGB6 with a topoisomerase I inhibitor payload. ITGB6 is overexpressed in many solid tumors, but shows minimal to no expression in most normal tissues, thereby potentially reducing the risk of systemic toxicity and off-target effects. SKB105 incorporates proprietary Kthiol® irreversible conjugation technology, linking an anti-ITGB6 fully human Immunoglobulin G1 (IgG1) monoclonal antibody (mAb) to a clinically validated cleavable linker. This design aims to enhance stability and tumor-specific payload delivery while reducing adverse effects. In preclinical models, SKB105 demonstrated a favorable efficacy, safety, and pharmacokinetic (PK) profile.
About SKB118(also known as CR-001)
SKB118/CR-001 is a tetravalent bispecific antibody being developed for the treatment of solid tumors that combines two complementary, validated mechanisms in oncology via a blockade of PD-1 and VEGF. PD-1 checkpoint inhibition is aimed at restoring T cells' ability to recognize and destroy tumor cells, and blocking VEGF is intended for reducing blood supply to tumor cells and inhibiting tumor growth. In preclinical studies, SKB118/CR-001 demonstrated cooperative pharmacology with increased binding to PD-1 and signal blockade in the presence of VEGF as well as robust anti-tumor activity. SKB118/CR-001's anti-VEGF activity may also normalize the vasculature at the tumor site, which has the potential to improve the localization and effectiveness of combination therapies, particularly in conjunction with ADCs.
About Kelun-Biotech
Kelun-Biotech (6990.HK) is a holding subsidiary of Kelun Pharmaceutical (002422.SZ), which focuses on the R&D, manufacturing, commercialization and global collaboration of innovative biological drugs and small molecule drugs. Kelun-Biotech focuses on major disease areas such as solid tumors, autoimmune, inflammatory, and metabolic diseases, and in establishing a globalized drug development and industrialization platform to address the unmet medical needs in China and the rest of world. Kelun-Biotech is committed to becoming a leading global enterprise in the field of innovative drugs. At present, Kelun-Biotech has more than 30 ongoing key innovative drug projects, of which 4 projects have been approved for marketing, 1 project is in the NDA stage and more than 10 projects are in the clinical stage. Kelun-Biotech has established one of the world's leading proprietary ADC and novel DC platforms, OptiDC™, and has 2 ADC projects approved for marketing, and multiple ADC and novel DC assets in clinical or preclinical research stage. For more information, please visit https://en.kelun-biotech.com/.
2026-01-05
CHENGDU, China, January 5, 2026 -- Sichuan Kelun-Biotech Biopharmaceutical Co., Ltd. (“Kelun-Biotech” or the “Company,” 6990.HK) today announced that its TROP2-directed antibody-drug conjugate (ADC) sacituzumab tirumotecan (sac-TMT, also known as SKB264/MK-2870) (佳泰莱®) in combination with MSD’s anti-PD-1 monoclonal antibody pembrolizumab (KEYTRUDA®[1]) was granted Breakthrough Therapy Designation (BTD) by the Center for Drug Evaluation (CDE) of the National Medical Products Administration (NMPA) of China for the first-line treatment of patients with locally advanced or metastatic non-small cell lung cancer (NSCLC) who have PD-L1 tumor proportion score (TPS)≥1% and are EGFR-negative and ALK-negative.
BTD is granted for treatment regimens that provide effective treatment or prevention for conditions with no currently available therapy, or that demonstrate significant clinical advantages over currently available treatments. For drugs included in the breakthrough therapy process, if relevant conditions are met, applications for conditional approval and priority review and approval can be submitted when applying for marketing authorization.
Previously, the company announced that results from the Phase III clinical trial of OptiTROP-Lung05, evaluating sac-TMT in combination with pembrolizumab as first-line treatment for PD-L1-positive NSCLC, demonstrated a statistically significant and clinically meaningful improvement in its primary endpoint of progression-free survival (PFS). A positive trend was also observed in overall survival (OS). OptiTROP-Lung05 is the first Phase III study of an immunotherapy and ADC combination to meet its primary endpoint in the first-line treatment of NSCLC. Granting of BTD for the first-line treatment of PD-L1-positive NSCLC indication offers pathways to expedite the review and potential approval process of sac-TMT for this indication.
To date, sac-TMT has received five BTDs for:
locally advanced or metastatic triple-negative breast cancer (TNBC) in July 2022;
locally advanced or metastatic EGFR-mutant NSCLC after progression on EGFR-TKI therapy in January 2023;
locally advanced or metastatic hormone-receptor positive (HR+) and human epidermal growth factor receptor 2-negative (HER2-) breast cancer (BC) in patients who have previously received at least two lines of systemic chemotherapy in June 2023;
first-line treatment of unresectable locally advanced, recurrent or metastatic PD-L1 negative TNBC in March 2024;
In combination with anti-PD-L1 monoclonal antibody tagitanlimab for the first-line treatment of locally advanced or metastatic non-squamous NSCLC without actionable genomic alterations in June 2025.
About sac-TMT
Sac-TMT, a core product of the Company, is a novel human TROP2 ADC in which the Company has proprietary intellectual property rights, targeting advanced solid tumors such as NSCLC, BC, gastric cancer (GC), gynecological tumors, among others. Sac-TMT is developed with a novel linker to conjugate the payload, a belotecan-derivative topoisomerase I inhibitor with a drug-to-antibody-ratio (DAR) of 7.4. Sac-TMT specifically recognizes TROP2 on the surface of tumor cells by recombinant anti-TROP2 humanized monoclonal antibodies, which is then endocytosed by tumor cells and releases the payload KL610023 intracellularly. KL610023, as a topoisomerase I inhibitor, induces DNA damage to tumor cells, which in turn leads to cell-cycle arrest and apoptosis. In addition, it also releases KL610023 in the tumor microenvironment. Given that KL610023 is membrane permeable, it can enable a bystander effect, or in other words kill adjacent tumor cells.
In May 2022, the Company licensed the exclusive rights to MSD (the tradename of Merck & Co., Inc, Rahway, NJ, USA) to develop, use, manufacture and commercialize sac-TMT in all territories outside of Greater China (which includes Mainland China, Hong Kong, Macao and Taiwan).
To date, three indications for sac-TMT have been approved and marketed in China for: EGFR mutant-positive locally advanced or metastatic non-squamous NSCLC following progression on EGFR-TKI therapy and platinum-based chemotherapy; Unresectable locally advanced or metastatic TNBC who have received at least two prior systemic therapies (at least one of them for advanced or metastatic setting); EGFR mutant-positive locally advanced or metastatic non-squamous NSCLC who progressed after treatment with EGFR-TKI therapy. The first two indications listed above have been included in China's National Reimbursement Drug List (NRDL). This inclusion is expected to bring clinical benefits to a greater number of cancer patients.
Sac-TMT is the world's first TROP2 ADC drug approved for marketing in lung cancer. In addition, the sNDA for sac-TMT for second-line and above treatment of HR+/HER2- BC was accepted by the Center for Drug Evaluation of the National Medical Products Administration, and was included in the priority review and approval process.
As of today, Kelun-Biotech has initiated 9 registrational clinical studies in China. MSD has initiated 15 ongoing Phase 3 global clinical studies of sac-TMT as a monotherapy or with pembrolizumab or other anti-cancer agents for several types of cancer. These studies are sponsored and led by MSD.
About Kelun-Biotech
Kelun-Biotech (6990.HK) is a holding subsidiary of Kelun Pharmaceutical (002422.SZ), which focuses on the R&D, manufacturing, commercialization and global collaboration of innovative biological drugs and small molecule drugs. Kelun-Biotech focuses on major disease areas such as solid tumors, autoimmune, inflammatory, and metabolic diseases, and in establishing a globalized drug development and industrialization platform to address the unmet medical needs in China and the rest of world. Kelun-Biotech is committed to becoming a leading global enterprise in the field of innovative drugs. At present, Kelun-Biotech has more than 30 ongoing key innovative drug projects, of which 4 projects have been approved for marketing, 1 project is in the NDA stage and more than 10 projects are in the clinical stage. Kelun-Biotech has established one of the world's leading proprietary ADC and novel DC platforms, OptiDC™, and has 2 ADC projects approved for marketing, and multiple ADC and novel DC assets in clinical or preclinical research stage. For more information, please visit https://en.kelun-biotech.com/.
[1] KEYTRUDA® (pembrolizumab) is a registered trademark of Merck Sharp & Dohme LLC (MSD), a subsidiary of Merck & Co., Inc., Rahway, NJ, USA.
2025-12-04
Companies to advance CR-001, a PD-1 x VEGF bispecific antibody, and SKB105, an integrin beta-6-directed antibody-drug conjugate (ADC), in global markets and China
Collaboration designed to accelerate and expand the development of synergistic combinations with CR-001 and ADCs, including SKB105
CR-001 and SKB105 on track to enter Phase 1/2 monotherapy clinical trials in Q1 2026 with combination studies to follow
CHENGDU, China and WALTHAM, Mass., Dec. 4, 2025 /PRNewswire/ -- Sichuan Kelun-Biotech Biopharmaceutical Co., Ltd. ("Kelun-Biotech", 6990.HK), which focuses on the R&D, manufacturing, commercialization and global collaboration of innovative biological drugs and small molecule drugs, and Crescent Biopharma, Inc. ("Crescent") (Nasdaq: CBIO), a biotechnology company dedicated to rapidly advancing the next wave of therapies for cancer patients, today announced that the companies have entered into a strategic partnership to develop and commercialize oncology therapeutics, including novel combinations.
The partnership involves Crescent's CR-001, a PD-1 x VEGF bispecific antibody, and Kelun-Biotech's SKB105, an integrin beta-6 (ITGB6)-directed antibody-drug conjugate (ADC) with a topoisomerase payload. Both candidates are being developed for the treatment of solid tumors and are expected to enter Phase 1/2 monotherapy clinical trials in the first quarter of 2026.
Under the terms of the collaboration, Crescent has granted Kelun-Biotech exclusive rights to research, develop, manufacture and commercialize CR-001 in Greater China (including mainland China, Hong Kong, Macau and Taiwan). In addition, Kelun-Biotech has granted Crescent exclusive rights to research, develop, manufacture and commercialize SKB105 in the United States, Europe and all other markets outside of Greater China. The partnership includes the development of these candidates as monotherapies, and also the evaluation of CR-001 in combination with SKB105.Both Crescent and Kelun-Biotech have the right to independently develop CR-001 in additional combinations, including combinations of CR-001 with proprietary ADC pipeline assets.
Dr. Michael Ge, chief Executive officer of Kelun-Biotech, said, "We are pleased to have entered into a partnership with Crescent for two innovative assets, CR-001 and SKB105. This collaboration complements and strengthens our differentiated oncology pipeline by the addition of CR-001 and also enables us to advance the development of SKB105 in the global market, bolstering its potential commercial value and our global collaboration network. Our creative global partnership combines the capabilities of both companies to explore novel monotherapies and combination strategies for tumor treatments with SKB105 and CR-001. By leveraging China's abundant clinical resources and Execution efficiency, we aim to expedite clinical development while rigorously maintaining the highest global standards. We believe this partnership creates a powerful synergy to maximize the potential of these two drug candidates for the treatment of patients in both China and the rest of the world."
"We are thrilled to be partnering with Kelun-Biotech, an established leader in the development and commercialization of ADCs who shares our commitment to bringing next generation therapeutics that can improve outcomes for people living with cancer," said Joshua Brumm, chief Executive officer of Crescent. "This collaboration expands our pipeline with the addition of SKB105, furthers our strategy of advancing multiple modalities across our portfolio, and accelerates our efforts to deliver synergistic combinations with CR-001, which has the potential to be a foundational backbone therapy. We look forward to working with Kelun-Biotech to drive innovative therapeutics with the potential to address multiple tumor types and transform cancer care."
Under the collaboration, Kelun-Biotech will receive an upfront payment of US$80 million from Crescent and is also eligible to receive additional milestones of up to US$1.25 billion, plus tiered middle single-digit to low double-digit royalties on net sales of SKB105. Kelun-Biotech is also eligible to receive additional payment from Crescent if Crescent undergoes a near-term change of control or enters into a sublicense agreement with a third party. Crescent will receive an upfront payment of US$20 million from Kelun-Biotech and is also eligible to receive additional milestones of up to US$30 million, plus tiered low to middle single digit royalties on net sales of CR-001.
About CR-001 (also known as SKB118)
CR-001 is a tetravalent bispecific antibody being developed for the treatment of solid tumors that combines two complementary, validated mechanisms in oncology via a blockade of PD-1 and VEGF. PD-1 checkpoint inhibition is aimed at restoring T cells' ability to recognize and destroy tumor cells, and blocking VEGF is intended for reducing blood supply to tumor cells and inhibiting tumor growth. In preclinical studies, CR-001 demonstrated cooperative pharmacology with increased binding to PD-1 and signal blockade in the presence of VEGF as well as robust anti-tumor activity. CR-001's anti-VEGF activity may also normalize the vasculature at the tumor site, which has the potential to improve the localization and effectiveness of combination therapies, such as the administration of CR-001 with antibody-drug conjugates (ADCs). A global Phase 1/2 trial of CR-001 in patients with solid tumors is anticipated to commence in the first quarter of 2026.
About SKB105 (also known as CR-003)
SKB105 is a differentiated ADC targeting integrin beta-6 (ITGB6) with a topoisomerase 1 inhibitor payload. ITGB6 is overexpressed in many solid tumors, but shows minimal to no expression in most normal tissues, thereby potentially reducing the risk of systemic toxicity and off-target effects. SKB105 consists of an anti-ITGB6 fully human IgG1 monoclonal antibody conjugated via a stable, clinically validated cleavable linker. The molecule incorporates proprietary Kthiol® irreversible conjugation technology, designed to enhance stability and tumor-specific payload delivery while reducing adverse effects. SKB105 demonstrated a favorable efficacy, safety, and pharmacokinetic (PK) profile in preclinical models. A Phase 1/2 clinical trial of SKB105 in patients with solid tumors is anticipated to commence in the first quarter of 2026.
About Kelun-Biotech
Kelun-Biotech (6990.HK) is a holding subsidiary of Kelun Pharmaceutical (002422.SZ), which focuses on the R&D, manufacturing, commercialization and global collaboration of innovative biological drugs and small molecule drugs. Kelun-Biotech focuses on major disease areas such as solid tumors, autoimmune, inflammatory, and metabolic diseases, and in establishing a globalized drug development and industrialization platform to address the unmet medical needs in China and the rest of world. Kelun-Biotech is committed to becoming a leading global enterprise in the field of innovative drugs. At present, Kelun-Biotech has more than 30 ongoing key innovative drug projects, of which 4 projects have been approved for marketing, 1 project is in the NDA stage and more than 10 projects are in the clinical stage. Kelun-Biotech has established one of the world's leading proprietary ADC and novel DC platforms, OptiDC™, and has 2 ADC projects approved for marketing, and multiple ADC and novel DC assets in clinical or preclinical research stage. For more information, please visit https://en.kelun-biotech.com/.
About Crescent Biopharma
Crescent Biopharma's vision is to build a world leading oncology company bringing the next wave of therapies for cancer patients. Crescent 's pipeline includes its lead program, a PD-1 x VEGF bispecific antibody, as well as novel antibody-drug conjugates (ADCs). By leveraging multiple modalities and established targets, Crescent aims to rapidly advance potentially transformative therapies either as single agents or as part of combination regimens to treat a range of solid tumors. For more information, visit www.crescentbiopharma.com and follow Crescent on LinkedIn and X.
2025-10-20
After a median follow-up of 18.9 months, the median PFS was 8.3 months in the sac-TMT group and 4.3 months in the chemotherapy group (hazard ratio (HR), 0.49; 95% confidence interval (CI), 0.39 to 0.62; two-sided P<0.0001).
OS was significantly longer with sac-TMT than with chemotherapy (HR, 0.60; 95% CI, 0.44 to 0.82; two-sided P=0.001); 18-month OS rate was 65.8% and 48.0%, respectively. In the supplementary analysis, in which data were censored at the start date of subsequent ADC, the HR was 0.56 (95% CI, 0.41 to 0.77).
Sac-TMT was associated with a higher incidence of stomatitis with most cases being mild and with grade 3 or higher cases reported in very few patients (4.8%; all were grade 3). Low incidence of ocular-surface toxic effects, no ILD or pneumonitis, and one infusion-related reaction (grade 2) was reported in the sac-TMT group.
On October 20, results from the Phase III registrational clinical study OptiTROP-Lung04—led by Professor Zhang Li's team from Sun Yat-sen University Cancer Center—were published in the New England Journal of Medicine (Impact Factor = 78.5). The study evaluated the efficacy and safety of the TROP2 antibody-drug conjugate (ADC) sacituzumab tirumotecan (sac-TMT) monotherapy versus pemetrexed plus platinum chemotherapy for the treatment of patients with epidermal growth factor receptor (EGFR)-mutant locally advanced or metastatic non-squamous non-small cell lung cancer (NSCLC) who have progressed failed after treatment with EGFR-tyrosine kinase inhibitor (TKI) therapy. The study was also selected as a Late-Breaking Abstract (LBA) at the 2025 European Society for Medical Oncology (ESMO) Congress and was presented as an oral report in the Presidential Symposium (Presentation # LBA5).
OptiTROP-Lung04 is a randomized, open-label, multicenter Phase III study designed to evaluate the efficacy and safety of sac-TMT monotherapy versus pemetrexed plus platinum in patients with EGFR-mutated locally advanced or metastatic NSCLC who had progressed on prior EGFR-TKI therapy. Eligible patients were those with histologically and/or cytologically confirmed disease. The primary endpoint was Progression-Free Survival (PFS) assessed by a Blinded Independent Review Committee (BIRC) per RECIST v1.1, and the key secondary endpoint was overall survival (OS). Results showed that compared with platinum-based doublet chemotherapy, sac-TMT monotherapy demonstrated a statistically significant and clinically meaningful improvement in both PFS and OS. Consistent PFS and OS benefits were observed across all prespecified subgroups, including prior EGFR-TKI therapy, presence of liver or brain metastases, and EGFR mutation subtype.
Based on the positive results of the OptiTROP-Lung04 study, sac-TMT has been approved by the National Medical Products Administration (NMPA) in China for the treatment of adult patients with EGFR mutation-positive locally advanced or metastatic non-squamous NSCLC who have progressed after EGFR-TKI therapy. To date, sac-TMT monotherapy remains the only approved treatment option for advanced EGFR-mutant NSCLC patients who have progressed after prior TKI therapy, as well as those who have progressed after prior TKI and platinum-based chemotherapy (either concurrently or sequentially). This achievement provides comprehensive coverage for the entire population of TKI-resistant patients.
Dr. Michael Ge, Chief Executive Officer of Kelun-Biotech, commented: “OptiTROP-Lung04 is a key study that propels sac-TMT from late-line to an earlier-line treatment-setting in lung cancer, achieving a significant improvement in overall survival. We are thrilled that the results have been published in The New England Journal of Medicine, allowing this important finding to be shared widely among medical professionals and providing a reference for future lung cancer research. Together with our partner MSD, we will continue to advance sac-TMT’s clinical development and regulatory approvals, covering broader lung cancer and other indications, with the goal of making sac-TMT a cornerstone therapy for the benefit of patients.”
Professor Shengxiang Ren from the Department of Oncology at Shanghai Pulmonary Hospital, Tongji University, stated: “The application of third-generation EGFR-TKIs has significantly improved the overall prognosis for patients with advanced EGFR-mutant NSCLC. However, drug resistance is almost inevitable. Traditional treatment after resistance involves platinum-based doublet chemotherapy, which offers limited overall benefit. In recent years, regimens of chemotherapy plus immune checkpoint inhibitors+anti-angiogenic agents, or EGFR/c-Met bsAb, or PD-1/VEGF bsAb, have been successively approved for this indication. However, all these approaches are chemotherapy-based, highlighting an urgent need to explore novel treatment regimens. The OptiTROP-Lung04 study confirms sac-TMT as the first treatment option as a monotherapy to deliver clear dual benefits in both PFS and OS following EGFR-TKI progression. The sequential application of sac-TMT prior to chemotherapy demonstrates strong competence, establishing itself as a new standard of care for patients with advanced EGFR-mutant lung cancer after TKI resistance.”
About Sac-TMT
Sac-TMT, a core product of the Company, is a novel human TROP2 ADC in which the Company has proprietary intellectual property rights, targeting advanced solid tumors such as NSCLC, breast cancer (BC), gastric cancer (GC), gynecological tumors, among others. Sac-TMT is developed with a novel linker to conjugate the payload, a belotecan-derivative topoisomerase I inhibitor with a drug-to-antibody-ratio (DAR) of 7.4. Sac-TMT specifically recognizes TROP2 on the surface of tumor cells by recombinant anti-TROP2 humanized monoclonal antibodies, which is then endocytosed by tumor cells and releases the payload KL610023 intracellularly. KL610023, as a topoisomerase I inhibitor, induces DNA damage to tumor cells, which in turn leads to cell-cycle arrest and apoptosis. In addition, it also releases KL610023 in the tumor microenvironment. Given that KL610023 is membrane permeable, it can enable a bystander effect, or in other words kill adjacent tumor cells.
In May 2022, the Company licensed the exclusive rights to MSD (the tradename of Merck & Co., Inc., Rahway, NJ, USA) to develop, use, manufacture and commercialize sac-TMT in all territories outside of Greater China (includes Mainland China, Hong Kong, Macau, and Taiwan).
To date, three indications for sac-TMT have been approved and marketed in China for the treatment of adult patients with unresectable locally advanced or metastatic triple negative breast cancer (TNBC) who have received at least two prior systemic therapies (at least one of them for advanced or metastatic setting), EGFR mutation-positive locally advanced or metastatic non-squamous NSCLC following progression on EGFR-TKI therapy and platinum-based chemotherapy and EGFR mutant-positive locally advanced or metastatic non-squamous NSCLC who progressed after treatment with EGFR-TKI therapy. Sac-TMT is the first TROP2 ADC drug approved for marketing in lung cancer globally. In addition, the new indication application for sac-TMT for the treatment of adult patients with unresectable locally advanced, metastatic hormone receptor positive (HR+) and human epidermal growth factor receptor 2-negative (HER2-) BC who have received prior endocrine therapy and other systemic treatments in the advanced or metastatic setting was accepted by the Center for Drug Evaluation of the NMPA, and was included in the priority review and approval process.
As of today, the Company has initiated 9 registrational clinical studies in China. MSD has initiated 15 ongoing Phase Ⅲ global clinical studies of sac-TMT as a monotherapy or with pembrolizumab1 or other anti-cancer agents for several types of cancer. These studies are sponsored and led by MSD.
About Kelun-Biotech
Kelun-Biotech (6990.HK) is a holding subsidiary of Kelun Pharmaceutical (002422.SZ), which focuses on the R&D, manufacturing, commercialization and global collaboration of innovative biological drugs and small molecule drugs. The company focuses on major disease areas such as solid tumors, autoimmune, inflammatory, and metabolic diseases, and in establishing a globalized drug development and industrialization platform to address the unmet medical needs in China and the rest of world. The Company is committed to becoming a leading global enterprise in the field of innovative drugs. At present, the Company has more than 30 ongoing key innovative drug projects, of which 4 projects have been approved for marketing, 1 project is in the NDA stage and more than 10 projects are in the clinical stage. The company has established one of the world's leading proprietary ADC and novel DC platforms, OptiDC™, and has 2 ADC projects approved for marketing, and multiple ADC and novel DC assets in clinical or preclinical research stage. For more information, please visit https://kelun-biotech.com/.
References:
1. Pembrolizumab (KEYTRUDA®) is a registered trademark of Merck Sharp & Dohme LLC (MSD), a subsidiary of Merck & Co., Inc., Rahway, NJ, USA.
2025-10-19
CHENGDU, China, Oct, 18 , 2025——Sichuan Kelun-Biotech Biopharmaceutical Co., Ltd. (the “Company”) announced that at the 2025 European Society for Medical Oncology (ESMO) Congress held in Berlin, Germany, Results from a Phase 3 study of the Company’s human epidermal growth factor receptor 2 (HER2)-directed ADC trastuzumab botidotin (also known as A166) trastuzumab botidotin versus trastuzumab emtansine (T-DM1) in HER2-positive unresectable or metastatic BC was presented as an oral report by Professor Xichun Hu from Fudan University Shanghai Cancer Center (Presentation # LBA24, Proffered paper session 1: Breast cancer, metastatic).
Trastuzumab botidotin is a HER2-targeted ADC composed of a cytotoxic drug (Duostatin-5, anti-microtubule agent) with site-specific conjugation to trastuzumab via a stable protease-cleavable valine-citrulline linker. The unique linker is stable in plasma and selectively cleaved by lysosomal cathepsin B that is up-regulated in cancer cells.
In this study, a total of 365 patients with HER2+ unresectable or metastatic BC who had received at least one prior anti-HER2 therapy were randomized (1:1) to receive trastuzumab botidotin or T-DM1. 53% of patients had received ≥2 lines of anti-HER2 therapy, 61% of patients had HER2 Immunohistochemistry (IHC) 3+, and 60% of patients had been treated with TKIs, particularly pyrotinib (56%). As of April 26, 2025, median follow-up was 14.9 months.
Median PFS was significantly longer in trastuzumab botidotin than in T-DM1 (11.1 months vs 4.4 months; HR: 0.39, 95% CI, 0.30-0.51, p<0.0001). PFS benefit with trastuzumab botidotin was consistently observed regardless of prior lines of anti-HER2 therapy (HR 0.36, 95% CI, 0.25-0.53, for 1 prior line; HR 0.39, 95% CI, 0.28-0.56, for ≥2 prior lines).
ORR by blinded independent central review (BICR) was 76.9% vs 53.0%.
A trend toward benefit in OS was observed in trastuzumab botidotin (HR 0.62; 95% CI, 0.38-1.03).
Grade ≥3 treatment emergent adverse events (TEAEs) occurred in 69.8% of patients in trastuzumab botidotin and 63.7% in T-DM1. The most common TEAEs associated with dose reduction were ocular AEs for trastuzumab botidotin, and were platelet count decreased for trastuzumab emtansine. Only two patients permanently discontinued trastuzumab botidotin due to TEAE. No on-treatment deaths were observed in trastuzumab botidotin, compared with 1.6% in T-DM1, all of which were considered unrelated to treatment.
As a conclusion, this second head-to-head trial comparing T-DM1 with other anti-HER2 regimens demonstrated that trastuzumab botidotin statistically improved PFS with an ORR of 76.9% vs 53.0%. PFS benefit with trastuzumab botidotin was consistently observed regardless of prior lines of anti-HER2 therapy. Ocular AEs were also manageable.
"Professor Xichun Hu, National Lead Principal Investigator from Fudan University Shanghai Cancer Center:“Trastuzumab botidotin effectively balances safety and efficacy through its unique molecular design, reducing the incidence of interstitial lung disease and hematologic toxicity. According to research data, trastuzumab botidotin demonstrated significant survival benefits in the pivotal Phase III trial, with an overall manageable safety profile, providing a new important treatment option for pretreated HER2+ BC patients. These positive results also offer robust evidence-based support for personalized treatment and updates to clinical practice guidelines.”
About Trastuzumab botidotin
Trastuzumab botidotin is a differentiated HER2 ADC to treat advanced HER2+ solid tumors. As an innovative HER2 ADC developed by the Company, it conjugates a novel, monomethyl auristatin F (MMAF) derivative (a highly cytotoxic tubulin inhibitor, Duo-5) via a stable, enzyme-cleavable linker to a HER2 monoclonal antibody with a DAR of 2. Trastuzumab botidotin specifically binds to HER2 on the surface of tumor cells and is internalized by tumor cells, releasing the toxin molecule Duo-5 inside the cell. Duo-5 induces tumor cell cycle arrest in the G2/M phase, leading to tumor cell apoptosis. After targeting HER2, trastuzumab botidotin can also inhibit the HER2 signaling pathway; it has antibody-dependent cell-mediated cytotoxicity (ADCC) activity.
Based on the results of a multi-center, randomized, open-label, controlled, Phase 3 KL166-III-06 study, trastuzumab botidotin was approved for marketing by the NMPA in for adult patients with unresectable or metastatic HER2 positive BC who have received one or more prior anti-HER2 therapy. At a pre-specified interim analysis, trastuzumab botidotin demonstrated a statistically significant and clinically meaningful improvement in the primary endpoint of PFS as assessed by the BICR compared with T-DM1[; the beneficial trend for OS of trastuzumab botidotin was also observed.
Currently, the Company has initiated an open, multi-center Phase 2 clinical study of trastuzumab botidotin in the treatment of HER2+ unresectable or metastatic BC that previously received a topoisomerase inhibitor ADC.
About Kelun-Biotech
Kelun-Biotech (6990.HK) is a holding subsidiary of Kelun Pharmaceutical (002422.SZ), which focuses on the R&D, manufacturing, commercialization and global collaboration of innovative biological drugs and small molecule drugs. The company focuses on major disease areas such as solid tumors, autoimmune, inflammatory, and metabolic diseases, and in establishing a globalized drug development and industrialization platform to address the unmet medical needs in China and the rest of world. The Company is committed to becoming a leading global enterprise in the field of innovative drugs. At present, the Company has more than 30 ongoing key innovative drug projects, of which 4 projects have been approved for marketing, 1 projects are in the NDA stage and more than 10 projects are in the clinical stage. The company has established one of the world's leading proprietary ADC and novel DC platforms, OptiDC™, and has 1 ADC project approved for marketing and multiple ADC and novel DC assets in clinical or preclinical research stage. For more information, please visit https://kelun-biotech.com/.
2026-01-07
The 44th J.P. Morgan Healthcare Conference (JPMHC) will be held in San Francisco, California, USA, from January 12 to 15, 2026. Sichuan Kelun-Biotech Biopharmaceutical Co., Ltd. (“Kelun-Biotech” or the “Company”, 6990.HK) has been invited to attend the conference and Dr. Michael Ge, President and CEO of Kelun-Biotech, will share the company’s latest business progresses as well as its innovation capabilities and strategies.
Presentation Time: Thursday, January 15, 2026 at 9:30 AM Pacific Standard Time
Presentation Venue: Westin St. Francis, San Francisco, USA
J.P. Morgan Annual Healthcare Conference is honored as a “benchmark for development and investment in the global healthcare area”. During the conference, Kelun-Biotech will join massive seasoned experts and industry leaders to discuss cutting-edge trends in biopharmaceutical innovation. Based on the ongoing transformation moment of the global pharmaceutical industry, the company will actively pursue mutually beneficial partnership opportunities on the international stage.
You can visit “Investor Relations - Investor Calendar” page of Kelun-Biotech’s official website for participation of live webcast of presentation session, and watch replay within 30 days after the conference.
About Kelun-Biotech
Kelun-Biotech (6990.HK) is a holding subsidiary of Kelun Pharmaceutical (002422.SZ), which focuses on the R&D, manufacturing, commercialization and global collaboration of innovative biological drugs and small molecule drugs. Kelun-Biotech focuses on major disease areas such as solid tumors, autoimmune, and metabolic diseases, and in establishing a globalized drug development and industrialization platform to address the unmet medical needs in China and the rest of world. Kelun-Biotech is committed to becoming a leading global enterprise in the field of innovative drugs. At present, Kelun-Biotech has more than 30 ongoing key innovative drug projects, of which 4 projects have been approved for marketing, 1 project is in the NDA stage and more than 10 projects are in the clinical stage. Kelun-Biotech has established one of the world's leading proprietary ADC and novel DC platforms, OptiDC™, and has 2 ADC projects approved for marketing, and multiple ADC and novel DC assets in clinical or preclinical research stage. For more information, please visit https://en.kelun-biotech.com/.
2025-10-18
CHENGDU, China, Oct, 19, 2025——Sichuan Kelun-Biotech Biopharmaceutical Co., Ltd. (the “Company”) announced that at the 2025 European Society for Medical Oncology (ESMO) Congress held in Berlin, Germany, results from a Phase 3 OptiTROP-Lung04 trial of the Company’s trophoblast cell-surface antigen 2 (TROP2)-directed antibody-drug conjugate (ADC) sacituzumab tirumotecan (sac-TMT) in EGFR-mutated non-small cell lung cancer (NSCLC) following progression on epidermal growth factor receptor-tyrosine kinase inhibitors (EGFR-TKIs) was presented as an oral report by Professor Li Zhang from Sun Yat-sen University Cancer Center (Presentation # LBA5, Presidential Symposium II) and were simultaneously published in the New England Journal of Medicine (Impact Factor = 78.5).
In the OptiTROP-Lung04 trial, a total of 376 patients were randomized (1:1) to receive sac-TMT monotherapy or chemotherapy.
As at the data cut-off date July 06, 2025, the median follow-up is 18.9 months. the median Progression-Free Survival (PFS) was 8.3 months in the sac-TMT group and 4.3 months in the chemotherapy group. Sac-TMT significantly improved PFS over chemotherapy with 51% lower risk of disease progression or death (hazard ratio (HR) 0.49; 95% confidence interval (CI), 0.39-0.62; P<0.0001).
At the preplanned interim analysis of overall survival (OS), the OS was not reached (NR) in the sac-TMT group and 17.4 months in the chemotherapy group. sac-TMT significantly improved OS over chemotherapy with 40% lower risk of death (hazard ratio (HR) 0.6; 95% CI: 0.44-0.82; two-sided P=0.001). In the supplemental analysis, when censoring patients at the date of initiation of subsequent ADCs, sac-TMT significantly improved OS over chemotherapy with 44% lower risk of death (HR, 0.56; 95% CI, 0.41 - 0.77).
Sac-TMT significantly improved ORR as compared to chemotherapy (60.6% vs 43.1%)
A consistent PFS and OS benefit of sac-TMT over chemotherapy was observed across all predefined subgroups, including prior EGFR-TKI therapy, presence of liver or brain metastases, and EGFR mutation subtype.
The incidence of any grade treatment-related adverse events (TRAEs) and grade ≥3 TRAEs was similar between the two groups. The most common TRAEs for both sac-TMT and chemotherapy were hematologic toxicities. No TRAEs led to discontinuation or death, and no cases of interstitial lung disease/pneumonitis were reported in the sac-TMT group. Ocular surface toxicity: occurred in 9.6% of patients in the sac-TMT group, all of which were grade 1 - 2.
As a conclusion, sac-TMT demonstrates highly statistically significant and clinically meaningful improvements in PFS and OS compared to platinum-based chemotherapy and showed a manageable safety profile, with no unexpected safety signals identified. Several global phase 3 studies of sac-TMT monotherapy (NCT06305754, NCT06074588) and combination study with osimertinib in China (NCT06670196) in EGFR-mutant NSCLC are ongoing.
Professor Zhang Li, National Lead Principal Investigator from Sun Yat-sen University Cancer Center, commented: "Compared to platinum-based doublet chemotherapy, sac-TMT not only significantly prolonged PFS but also demonstrated a statistically significant and clinically meaningful improvement in OS within this patient population. This achievement marks a major breakthrough in global lung cancer treatment—sac-TMT, as a monotherapy, demonstrated statistically significant and clinically meaningful improvements in both PFS and OS in the Phase III trial for patients with EGFR-TKI-resistant NSCLC. This study provides highly valuable, new evidence-based guidance for lung cancer management worldwide and has the potential to reshape the therapeutic landscape for EGFR-TKI-resistant NSCLC "
About sac-TMT
Sac-TMT, a core product of the Company, is a novel human TROP2 ADC in which the Company has proprietary intellectual property rights, targeting advanced solid tumors such as NSCLC, BC, GC, gynecological tumors, among others. Sac-TMT is developed with a novel linker to conjugate the payload, a belotecan-derivative topoisomerase I inhibitor with a drug-to-antibody-ratio (DAR) of 7.4. Sac-TMT specifically recognizes TROP2 on the surface of tumor cells by recombinant anti-TROP2 humanized monoclonal antibodies, which is then endocytosed by tumor cells and releases the payload KL610023 intracellularly. KL610023, as a topoisomerase I inhibitor, induces DNA damage to tumor cells, which in turn leads to cell-cycle arrest and apoptosis. In addition, it also releases KL610023 in the tumor microenvironment. Given that KL610023 is membrane permeable, it can enable a bystander effect, or in other words kill adjacent tumor cells.
In May 2022, the Company licensed the exclusive rights to MSD (the tradename of Merck & Co., Inc, Rahway, NJ, USA) to develop, use, manufacture and commercialize sac-TMT in all territories outside of Greater China (which includes Mainland China, Hong Kong, Macao and Taiwan).
To date, three indications for sac-TMT have been approved and marketed in China for the treatment of adult patients with unresectable locally advanced or metastatic triple negative breast cancer (TNBC) who have received at least two prior systemic therapies (at least one of them for advanced or metastatic setting), EGFR mutation-positive locally advanced or metastatic non-squamous NSCLC following progression on EGFR-TKI therapy and platinum-based chemotherapy and EGFR mutant-positive locally advanced or metastatic non-squamous NSCLC who progressed after treatment with EGFR-TKI therapy. Sac-TMT is the first TROP2 ADC drug approved for marketing in lung cancer globally. In addition, the new indication applications for sac-TMT for the treatment of adult patients with unresectable locally advanced, metastatic HR+/HER2- BC who have received prior endocrine therapy and other systemic treatments in the advanced or metastatic setting was accepted by the Center for Drug Evaluation (CDE) of the National Medical Products Administration (NMPA), and was included in the priority review and approval process.
As of today, the Company has initiated 9 registrational clinical studies in China. MSD has initiated 15 ongoing Phase 3 global clinical studies of sac-TMT as a monotherapy or with pembrolizumab or other anti-cancer agents for several types of cancer. These studies are sponsored and led by MSD.
About Kelun-Biotech
Kelun-Biotech (6990.HK) is a holding subsidiary of Kelun Pharmaceutical (002422.SZ), which focuses on the R&D, manufacturing, commercialization and global collaboration of innovative biological drugs and small molecule drugs. The company focuses on major disease areas such as solid tumors, autoimmune, inflammatory, and metabolic diseases, and in establishing a globalized drug development and industrialization platform to address the unmet medical needs in China and the rest of world. The Company is committed to becoming a leading global enterprise in the field of innovative drugs. At present, the Company has more than 30 ongoing key innovative drug projects, of which 4 projects have been approved for marketing, 1 project is in the NDA stage and more than 10 projects are in the clinical stage. The company has established one of the world's leading proprietary ADC and novel DC platforms, OptiDC™, and has 2 ADC project approved for marketing and multiple ADC and novel DC assets in clinical or preclinical research stage. For more information, please visit https://kelun-biotech.com/.
2025-10-18
CHENGDU, China, Oct, 18 , 2025——Sichuan Kelun-Biotech Biopharmaceutical Co., Ltd. (the “Company”) announced that at the 2025 European Society for Medical Oncology (ESMO) Congress held in Berlin, Germany, results from a Phase 3 OptiTROP-Breast02 study of the Company’s trophoblast cell-surface antigen 2 (TROP2)-directed antibody-drug conjugate (ADC) sacituzumab tirumotecan (sac-TMT) (佳泰莱®) in previously treated locally advanced or metastatic hormone receptor-positive (HR+) and human epidermal growth factor receptor 2 -negative (HER2-) breast cancer (BC) was presented as an oral report by Professor Man Li from the Second Affiliated Hospital of Dalian Medical University (Presentation # LBA23, Proffered paper session 1: Breast cancer, metastatic). Previously, the new indication applications for sac-TMT for this indication was accepted by the National Medical Products Administration (NMPA) and was included in the priority review and approval process.
In the OptiTROP-Breast02 study, a total of 399 patients with HR+/HER2- BC who had progression on CDK4/6 inhibitors and received at least one prior line of chemotherapy in the advanced/metastatic setting were randomized (1:1) to receive sac-TMT or investigator's choice of chemotherapy (ICC).
As at the data cut-off date January 22, 2025, the median PFS was significantly longer in sac-TMT than in ICC (8.3 vs. 4.1 months; HR, 0.35; 95% CI, 0.26-0.48; P<0.0001). Clinical benefit was seen in sac-TMT independent of HER2 expression (HR for PFS in HER2-zero: 0.39, 95% CI, 0.26-0.57; in HER2-low: 0.31, 95% CI, 0.20-0.48).
Sac-TMT showed longer DoR versus chemotherapy; and ORR was also superior with sac-TMT to ICC.
There was a trend in OS that favored sac-TMT over ICC (HR, 0.33; 95% CI, 0.18-0.61).
Grade ≥ 3 TRAEs occurred in 62.0% and 64.8% of patients in sac-TMT and ICC. TRAE led to discontinuation in 0% and 0.5% of patients; pneumonitis occurred in 1.5% and 1.0% of patients (all grade 1-2) in sac-TMT and ICC, respectively.
As a conclusion, sac-TMT demonstrated statistically significant and clinically meaningful improvement in PFS compared to chemotherapy. PFS benefit was observed across all predefined subgroups and independent of HER2 status. Sac-TMT also showed a trend toward OS benefit (HR, 0.33; 95% CI, 0.18, 0.61) and demonstrated a favorable and manageable safety profile, with no new safety signals. Currently, a Phase III clinical study of sacituzumab tirumotecan as monotherapy and in combination with pembrolizumab in patients with chemotherapy-naïve HR+/HER2- breast cancer is ongoing globally, with a planned enrollment of approximately 1,200 participants. Additionally, another Phase III registrational study in the same population is currently enrolling patients in China, with a planned enrollment of 430 participants.
Professor Man Li from the Second Affiliated Hospital of Dalian Medical University, stated:“The Phase III OptiTROP-Breast02 study confirmed that, regardless of HER2 expression status, sac-TMT demonstrated promising efficacy in previously treated HR+/HER2- BC patients. As the first Phase III clinical trial focusing exclusively on an all-Chinese population with HR+/HER2- BC, the presentation of the OptiTROP-Breast02 study data at ESMO marks a significant breakthrough in clinical research in this indications in China. The positive outcomes of this study not only provide evidence-based support for the treatment of HR+/HER2- BC but also offer a safe and reliable new treatment option for breast cancer patients.”
About sac-TMT (佳泰莱®)
Sac-TMT, a core product of the Company, is a novel human TROP2 ADC in which the Company has proprietary intellectual property rights, targeting advanced solid tumors such as NSCLC, BC, GC, gynecological tumors, among others. Sac-TMT is developed with a novel linker to conjugate the payload, a belotecan-derivative topoisomerase I inhibitor with a drug-to-antibody-ratio (DAR) of 7.4. Sac-TMT specifically recognizes TROP2 on the surface of tumor cells by recombinant anti-TROP2 humanized monoclonal antibodies, which is then endocytosed by tumor cells and releases the payload KL610023 intracellularly. KL610023, as a topoisomerase I inhibitor, induces DNA damage to tumor cells, which in turn leads to cell-cycle arrest and apoptosis. In addition, it also releases KL610023 in the tumor microenvironment. Given that KL610023 is membrane permeable, it can enable a bystander effect, or in other words kill adjacent tumor cells.
In May 2022, the Company licensed the exclusive rights to MSD (the tradename of Merck & Co., Inc, Rahway, NJ, USA) to develop, use, manufacture and commercialize sac-TMT in all territories outside of Greater China (which includes Mainland China, Hong Kong, Macao and Taiwan).
To date, three indications for sac-TMT have been approved and marketed in China for the treatment of adult patients with unresectable locally advanced or metastatic triple negative breast cancer (TNBC) who have received at least two prior systemic therapies (at least one of them for advanced or metastatic setting), EGFR mutation-positive locally advanced or metastatic non-squamous NSCLC following progression on EGFR-TKI therapy and platinum-based chemotherapy and EGFR mutant-positive locally advanced or metastatic non-squamous NSCLC who progressed after treatment with EGFR-TKI therapy. Sac-TMT is the first TROP2 ADC drug approved for marketing in lung cancer globally. In addition, the new indication applications for sac-TMT for the treatment of adult patients with unresectable locally advanced, metastatic HR+/HER2- BC who have received prior endocrine therapy and other systemic treatments in the advanced or metastatic setting was accepted by the Center for Drug Evaluation (CDE) of the National Medical Products Administration (NMPA), and was included in the priority review and approval process.
As of today, the Company has initiated 9 registrational clinical studies in China. MSD has initiated 15 ongoing Phase 3 global clinical studies of sac-TMT as a monotherapy or with pembrolizumab or other anti-cancer agents for several types of cancer. These studies are sponsored and led by MSD.
About Kelun-Biotech
Kelun-Biotech (6990.HK) is a holding subsidiary of Kelun Pharmaceutical (002422.SZ), which focuses on the R&D, manufacturing, commercialization and global collaboration of innovative biological drugs and small molecule drugs. The company focuses on major disease areas such as solid tumors, autoimmune, inflammatory, and metabolic diseases, and in establishing a globalized drug development and industrialization platform to address the unmet medical needs in China and the rest of world. The Company is committed to becoming a leading global enterprise in the field of innovative drugs. At present, the Company has more than 30 ongoing key innovative drug projects, of which 4 projects have been approved for marketing, 1 project is in the NDA stage and more than 10 projects are in the clinical stage. The company has established one of the world's leading proprietary ADC and novel DC platforms, OptiDC™, and has 2 ADC project approved for marketing and multiple ADC and novel DC assets in clinical or preclinical research stage. For more information, please visit https://kelun-biotech.com/.
2024-05-20
More
On May 16, 2024, Kelun Group celebrated the Group's twenty-eighth birthday. On the occasion of the 28th birthday of Kelun Group, Kelun-Biotech (6990.HK), together with Kelun Research Institute, honored the long-serving employees and celebrated with them. The "28 years of riding the wind together, I grow with Kelun" series of staff activities came to a successful conclusion.
Activity 1: "Cultural Symbiosis" Theme Activity
The "Cultural Symbiosis" activity began with the firm and powerful "Elite Oath" of 170 employees from 34 teams. In the "Culture Lecture - Side by Side - Culture Transmission - Working Together - Dedication to Factory Day" competition, colleagues within the team support each other to work together, the teams learn from each other to catch up with each other, in a pleasant and intense atmosphere to promote the corporate culture, enhance the team cohesion, and match the style of the people of Kelun!

Highlights of the event


Activity 2: "All the Way" Long-term Service Employee Recognition Ceremony
To fight for the waves, a group of Kelun people have been working together with great expertise. In order to inspire and thank the long-serving employees of Kelun, on May 16th, the Group's birthday, Kelun-Biotech and Kelun Research Institute honored more than 40 employees who have joined Kelun Group for 15 years and above.
At the recognition ceremony, the company management presented each long-service employee with a certificate of honor and a souvenir. Employees are one of the most valuable assets of Kelun, they have traveled with Kelun all the way, not only witnessed the growth of the company, but also promoted the development of the company so far.
Kelun-Biotech also looks forward to more employees joining the ranks of long-term service in the future, witnessing the growth of Colum, meeting more challenges and opportunities together with the company, and becoming the backbone of promoting the development of the company.
"Twenty-eight years of riding the wind together, I grow up with Kelun", all the staff of Kelun-Biotech will be with good wishes for Kelun, and with the company to sail the waves together to make a new chapter!
2024-05-16
More

2024-05-04
More
(stock code: 6990.HK, hereinafter referred to as "Kelun-Biotech" or the "Company") has received approval from the U.S. Food and Drug Administration (FDA) to conduct Phase 2 clinical trials for its main product, A400 (also known as KL590586 or EP0031). (also known as KL590586 or EP0031), the Company's main product, has been approved by the U.S. Food and Drug Administration (FDA) for Phase 2 clinical trials.

A400 (EP0031) is a second-generation selective RET inhibitor (SRI) with broad activity against common RET gene fusions and mutations, designed to address clinical needs not met by first-generation SRIs. Targeting RET-driven cancers, next-generation SRIs offer potentially more diverse treatment options and may further improve patient prognosis.
In March 2021, Kelun-Biotech granted an exclusive, paid-up license to Ellipses Pharma Limited ("Ellipses"), a UK-based pharmaceutical company, to develop, manufacture and commercialize A400 (EP0031) in all countries outside of Greater China, North Korea, South Korea, Singapore, Malaysia and Thailand. (EP0031) in all countries except Greater China, Korea, South Korea, Singapore, Malaysia and Thailand.
In June 2022, A400 (EP0031) received FDA approval of an Investigational New Drug (IND) application for a Phase 1/2 trial in patients with RET-altered malignancies.
In November 2023,A400 was granted orphan drug status designation by the FDA for the treatment of RET fusion-positive solid tumors.
In March 2024, A400 was granted Fast Track designation by the FDA for the treatment of RET fusion-positive non-small cell lung cancer (NSCLC).
Currently, Kelun-Biotech is conducting a pivotal clinical study of A400 (EP0031) in China in RET-positive NSCLC.
In preclinical studies, A400 (EP0031) demonstrated favorable inhibitory activity against major RET kinases in vitro and in vivo, and A400 (EP0031) also demonstrated good blood-brain barrier penetration in animal models.
Data on A400 (EP0031) shared at the 2023 American Society of Clinical Oncology (ASCO) Annual Meeting showed that, based on the results of its ongoing Phase 1/2 trial, A400 (EP0031) demonstrated good anti-tumor efficacy in patients with advanced RET+ solid tumors, particularly in first- and second- or higher-line advanced RET+ NSCLC, with ORRs of 80.8% and 69.7%. The DCR in both cases was reported to be more than 96%.
At the upcoming 2024 ASCO Annual Meeting, the Company's partner Ellipses will report clinical data from the A400 (EP0031) Phase 1 dose-escalation and extension study conducted in patients with advanced RET variant NSCLC and other oncology treatments who have never been treated with an SRI or prior therapy on June 3, 2024 (local time).
Kelun-Biotech is committed to developing innovative medicines with features and advantages to address global unmet clinical needs, and the company will also accelerate the development of the drug in China and work with its partner Ellipses to advance the global development and commercialization of A400 (EP0031) to benefit more oncology patients around the world.
About Kelun-Biotech
Kelun-Biotech(6990.HK)is a holding subsidiary of Kelun Pharmaceutical (002422.SZ), which focuses on the R&D, manufacturing, commercialization and global collaboration of innovative biological drugs and small molecule drugs. The company focuses on major disease areas such as solid tumors, autoimmune, inflammatory, and metabolic diseases, and in establishing a globalized drug development and industrialization platform to address the unmet medical needs in China and the rest of world. The Company is committed to becoming a leading global enterprise in the field of innovative drugs.
At present, the Company has more than 30 ongoing innovative projects in major disease areas such as solid tumors, autoimmune, inflammatory, and metabolic diseases, including over 10 projects in the clinical stage and 4 projects in the NDA stage with several global trials being conducted simultaneously in multiple countries, including China, Europe, and the United States. The company has established one of the world’s leading proprietary ADC platforms, OptiDC?, and has 5 ADC projects in the clinical stage (2 of which are in the NDA stage) and several projects in the preclinical stage. For more information, please visit https://kelun-biotech.com/.
2024-04-12
More

In this beautiful season of "wet with apricot blossom rain, not cold with willow wind", the work conference of 2024 Kelun-Biotech was grandly held in Chengdu from April 10th to 12th. Mr. Liu Gexin, Chairman of the Board, Mr. Liu Sichuan, General Manager of the Group, senior management team, relevant persons in charge of each subsidiary (branch) company, each marketing area and all departments directly under the company attended the meeting, and at the same time, the whole meeting was broadcasted in the form of video, with a total number of more than 4,000 people attending the meeting. According to the agenda of the meeting, the 11th was synchronized with the meeting of the industrial sub-groups and marketing sub-groups, and the morning of the 12th was the working conference of the group. A total of 39 speakers made presentations and focused on the short-term and future trends of production and marketing.
The year of 2023 is the year when Kelun resolutely implements the "Twenty Character Guidelines" and continues to move forward victoriously, and the vision and courage of all the fighters extend the road of success of Kelun here. The successful spin-off and listing of Chuanning Biologicals and Kelun-Biotech, Kelun has formed a "character" structure operation platform, and as of March 2024, the total market capitalization has exceeded 100 billion yuan, becoming a pioneering force to lead the industry's high-quality development.
The industrial sub-group meeting focused on safety, quality, cost and other core themes. Kelun Drug Research Institute, Xindu Base, Hunan Kelun, Yueyang Branch, Hubei Kelun, Qionglai Branch focused on research and production linkage, production and marketing linkage, intelligent manufacturing, and high-quality development were comprehensively summarized.
The EHS Supervision Department, a department directly under the headquarters, focused on problems, prevented small steps, strengthened the construction of the safety system and the implementation of responsibilities, and continued to optimize the safety system and strengthen the safety culture with the help of PMS and other management tools. The Cost Management Committee put forward the company's thoughts and requirements on cost management from optimizing cost management, efficient linkage cost reduction, etc., and formulated a cost-saving incentive program, which injected a powerful agent for the production enterprises to continuously reduce costs and increase efficiency. The General Manager's Department made an in-depth summary around the dimensions of solidifying the quality cornerstone, creating an economic supply center, innovation empowerment, and improving quality and efficiency, and made important work plans and deployments in promoting industrial upgrading, and creating a competitive advantage in quality and cost.
The agenda of the meeting was also interspersed with thematic discussion sessions, for the current hotspots and production quality management practices, Internet + safety production, material key attribute assessment and control, supplier control strategy, artificial intelligence and other in-depth discussions, clarifying the management ideas, clearing the direction of the promotion and strategy.
At the end of the theme report, Mr. Liu Gexin, the chairman of the board of directors, commented on the speeches of each theme and pointed out that: soldiers are precious and quick! In the current market environment, through the adjustment of industrial structure, rapid release of production capacity, stabilize market expectations; follow the "Hein's Law", cast product quality shield.
Since its establishment in 2021, under the guidance of the company's "twenty-word policy", Kelun Marketing Center has further improved the company's operational efficiency with reform thinking and innovative approaches. At present, the marketing center is at the intersection of two three-year plans, this meeting comprehensively summarized the first three-year plan and comprehensively deployed the tasks of the second three-year plan.
Core Business Marketing Division, New Drug Marketing Division, Kelun-Biotech Marketing Center, CDMO Business Development Department, Comprehensive Products Division and Large Retail Division analyzed and summarized the sales and market situation of the company's core infusion products and generic innovative products from 2021 to 2023, and put forward the sales planning for 2024 and the long term; Health Strategy Development Research Department, County Business Department, Merchants Department and excellent areas shared the system construction, academic promotion and other multi-dimensional work experience. The Market Access Department, the Integrated Medical Market Department and the Business Department made special reports on the layout of access work, the professional capacity and system construction of academic promotion, and the management of product channels.

After listening carefully to the special report of each spokesman of the marketing sub-group, General Manager of the Marketing Center Fan Wendi made an in-depth and detailed comment, and deployed the second three-year plan of the Marketing Center - Deepening Reform, Steady Transformation, from the four dimensions of organizational optimization and talent cultivation, deepening of the business and comprehensive integration, improving the effectiveness and compliance transformation, and opportunities and problems. He also pointed out that China's pharmaceutical industry has gone through the dilemma of "no drugs available", the market-oriented operation of pulling up seedlings to help them grow, the difficult period of domestic substitution of generic drugs, and the agitation under the innovation dividend, and is now moving towards rationality and maturity. Every day, we must find the long-term trend in the changes and explore new breakthroughs in the unchanged!
Chairman Liu Gexin instructed: the top management has reached a high degree of consensus on the upgrading and transformation of marketing; the ice has been broken, the course has been indicated, and the road has been opened, and we are going to take resolute actions to quickly reach the expected reform goals.

Mr. Deng Xuheng, General Manager of Chuanning Bio, said: Chuanning adheres to the innovative development concept of building industrial alliance in known fields and forming knowledge alliance in unknown fields, and has been at the forefront of the industry in terms of industrial scale, product quality, environmental protection and governance level, and has become the head producer with international discourse power in bulk pharmaceutical raw materials. After listing, Chuanning has focused its resources on the synthetic biology track and completed the research and development of its first synthetic biology product, red myrcene glycol, to commercial production in two years, which fully demonstrated Chuanning's high-quality research and development level and strong industrialization capability. In the future, Chuanning will continue to adhere to the dual-wheel drive strategy of "bio-fermentation" and "synthetic biology", empowering the antibiotic industry and synthetic biology R&D with AI to further consolidate the head position of the traditional antibiotic industry, and at the same time accelerating the research and development and landing of new products, which will contribute to the profitability of Coren Group. At the same time, we will accelerate the research and development of new products, and contribute to the sustainable growth of the profit of Kelun.

On behalf of Kelun Research Institute and Kelun-Biotech, Mr. Ge Junyou, General Manager of Kelun-Biotech, reported the progress of Kelun's drug R&D work in 2023 and the work objectives in 2024, taking into account the current domestic and international regulations and competitive situation. He said: At present, the challenges and opportunities of new drug R&D coexist, Kelun will continue to adhere to the R&D strategy of "Generic drives innovation, innovation drives the future", under the strong and wise leadership of the Chairman, General Manager and the Group, continue to strengthen the full-cycle empowerment of innovative drug R&D by AI technology, focus on key indications and core technology platforms, fully promote international cooperation, and accelerate the development of multiple innovative drugs, as well as the development of new drugs in the future. We will also make every effort to promote international cooperation, accelerate the approval and commercialization of many innovative drugs, maximize the global value of our pipeline, and contribute China's power to the cause of human health!
The awarding ceremony of "The Fifth Group Meeting of Kelun Group" was also held during the meeting, and the top ten outstanding marketing areas in terms of net income and per capita profit for the year of 2023, and the outstanding production enterprises in the "Billion Dollar Club" were awarded respectively. The loyal and brave generals of Kelun marketing business face the challenges with the mindset of accommodating all rivers and the confidence of never giving up; the team will be cohesive as one and the spirit of struggle to achieve the mission frequently show their swords, which is so awe-inspiring and courageous! "It seems to be the most rugged, into as easy but hard", Colum this growing, growing production and technology team, is the foundation and hope for the continued success of the Colum business; it is a group of people who must be cherished and cared for, no matter how it is evaluated will not be too high. We are bold and confident in realizing this year's goal! Let us work together to open a new era of carrying the glory of Kelun, move forward!

Chairman Liu Gexin attended the whole meeting, and after listening to the speeches on industry, marketing, Chuanning Biological and R&D innovation, he put forward opinions and suggestions in light of the actual work and responded to the concerns of all parties.
Chairman Liu Gexin talked about: a bird dares to sing in the fragile branch, why it does not worry about the branch will break? Because it has wings to fly, the enterprise is the same, from the moment of the birth of Kelun, the eagle is our spiritual totem, through the clouds and fog, the eyes of heaven and earth, "Kunpeng wings, ninety thousand miles, turn the shaking goat's horns", which is the ambition of Kelun.
After twenty-eight years of hard work, Coren is at a "high time" that has never been seen before in its history. We must be virtuous and prudent, cautious and fearful; we must adhere to long-termism and value investment, and become a trustworthy company by rewarding investors and society with more excellent results.
In the face of the great changes that have not been seen in a century, embracing change from the bottom of our hearts is the only way to see the opportunities in economic adjustments and business changes. The only way to cope with change is to innovate, which is the essence of entrepreneurship. Success lies not only in talent and luck, but also in the anticipation and selection of quality "tracks", which requires entrepreneurs to have higher wisdom and commitment to explain the present and foresee the future with new knowledge systems and deeper insights. Entrepreneurs' belief in technology, their pursuit of innovation and their confidence in the future are intertwined, and these are great enablers for creating a new era of business.
Whenever we start a new impact, we will seek the regeneration power of "Phoenix Nirvana" from the original civilization of Colum's entrepreneurship, and the spirit of Colum, which is mutually promoting and stimulating, has been honed through the vicissitudes of life and has been deposited in the hearts of Colum's comrades, and will explode when there is a fire.
Today, we are standing on a new starting point, has a completely different state of mind. Warm winds of May will soon blow across the land of China, this is the season belonging to us, since we have chosen a faraway place, we will travel through thick and thin, let's sing for the month of May, and pay homage to those who struggle!
2024-04-08
More
2024 American Association for Cancer Research (AACR) Annual Meeting is being held in San Diego, California, the United States of America from April 5th to 10th, 2024, local time.

Sichuan Kelun-Biotech Biopharmaceutical Co., Ltd. (6990.HK, the “Company”) will present two study results of anti-TROP2 ADC sacituzumab tirumotecan (sac-TMT, formerly SKB264/MK-2870) during the AACR meeting scheduled below. The abstracts for the studies have been published on the official website of the AACR on April 5th, 2024, local time (See link below).
1. The updated efficacy and safety results for its anti-TROP2 ADCSKB264/sac-TMT in patients with previously treated advanced non-small cell lung cancer (NSCLC) from a phase 2 study in a poster session scheduled on April 9 2024, 1:30 PM - 5:00 PM local time (Abstract Presentation Number: CT247).
2.The preliminary efficacy and safety results for its anti-TROP2 ADC SKB264/sac-TMT in patients with previously treated advanced gastric or gastroesophageal junction (GEJ) cancer from a phase 2 study as an oral presentation, which is scheduled in a session on April 9 2024, 2:30 PM - 4:30 PM local time (Abstract Presentation Number: CT038).

The study results are summarized as follows:
NSCLC
Patients with previously treated advanced NSCLC were enrolled to receive SKB264/sac-TMT at 5 mg/kg Q2W until disease progression or unacceptable toxicity (KL264-01, NCT04152499). The data cut-off date was November 22, 2023.
Five Phase 3 global studies of SKB264/sac-TMT in patients with NSCLC are ongoing. Including two Phase 3 global studies of SKB264/sac-TMT in patients with 3L+ EGFR mutant NSCLC (NCT06074588), and 2L EGFR mutant NSCLC (NCT06305754) and a Phase 3 study ofSKB264/sac-TMT in China in patients with 2L EGFR mutant NSCLC (NCT05870319). Additionally two Phase 3 global studies of SKB264/sac-TMT plus pembrolizumab in patients with metastatic NSCLC expressing programmed death ligand 1 (PD-L1)≥ 50% (NCT06170788) and resectable NSCLC not achieving pathological complete response (NCT06312137) are ongoing.
As of the Data Cut-off date, 43 NSCLC patients had been enrolled and the median follow-up was 17.2 months. 21 patients with EGFR wild type had received a median of 3 prior regimens of therapy including anti-PD-1/L1 inhibitors. 22 patients with EGFR mutant had progressed on or after TKI therapy, 50% of whom also failed at least one line of chemotherapy. Updated efficacy results are shown in the following:
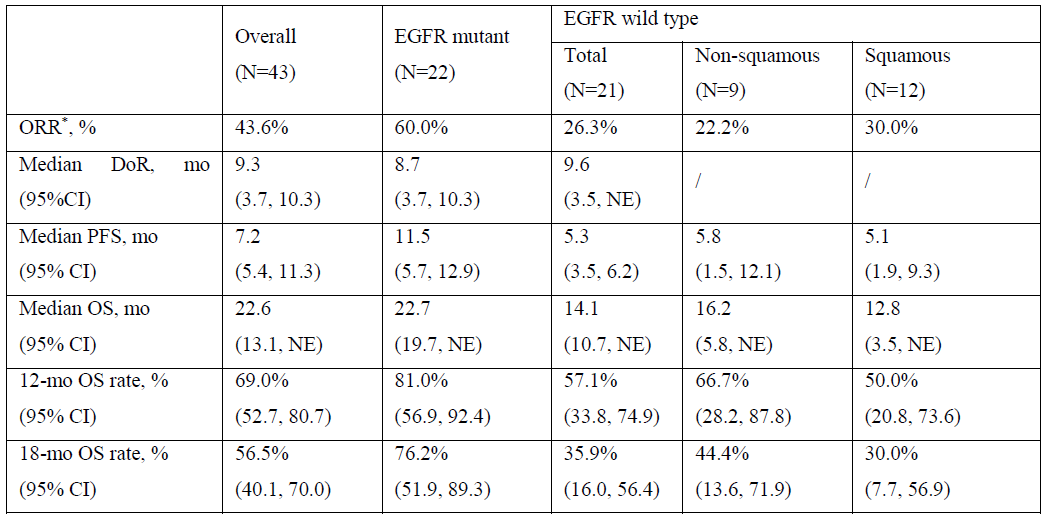
*Including confirmed or unconfirmed response. Based on response evaluable patients (≥1 on-study scans) with 4 patients (2 EGFR mutant patients with non-squamous histology and 2 EGFR wild type patients with squamous histology) excluded.
The most common Grade ≥3 treatment-related adverse events (TRAEs) were neutrophil count decreased (34.9%), anemia (30.2%), white blood cell (WBC) count decreased (25.6%), stomatitis (9.3%), and rash (7.0%). No TRAEs leading to treatment discontinuation or deaths occurred. No drug-related interstitial lung disease (ILD)/pneumonitis was reported.
Gastric/GEJ cancer
Patients with previously treated inoperable advanced gastric/GEJ adenocarcinoma were enrolled to receive SKB264/sac-TMT monotherapy at 5 mg/kg Q2W until disease progression or unacceptable toxicity in Phase 2 expansion cohort of KL264-01 study (NCT04152499). Patients with heavily pre-treated gastric/GEJ cancer were enrolled first, and then the cohort was amended to enroll patients with only one prior therapy of chemotherapy and anti-PD-1/L1 therapy. The data cut-off date was Nov 22, 2023.
As of the data cut-off date, a total of 48 patients were enrolled and followed up for at least 9 weeks. 24 patients (50.0%) had received one prior line of therapy (2L), while 24 patients (50.0%) had received ≥ 2 prior lines of therapy (3L+). 40 patients (83.3%) had received prior anti-PD-1/L1 inhibitors. Of 41 response-evaluable patients (defined as ≥ 1 on-study scans), the objective response rate (ORR) was 22.0% (9 partial responses, 2 pending confirmation) and disease control rate (DCR) was 80.5%. The ORRs in the 2L and 3L+ setting were 27.3% (including 2 pending confirmation) and 15.8%, respectively. Median duration of response (DoR) was 7.5 months. In the subset of 3L+ patients (n=24 including 54.2% of patients with ≥ 4 prior lines of therapy) with more mature follow-up (median follow up of 14.6 months), the median progression free survival (mPFS) was 3.7 months (95% CI: 2.6, 5.4) and median overall survival (mOS) was 7.6 months (95% CI: 5.3, 15.5).
The most common ≥ Grade 3 TRAEs were anemia (20.8%), neutrophil count decreased (18.8%), WBC decreased (12.5%) and neutropenia (6.3%). No TRAEs leading to treatment discontinuation or deaths occurred. No neuropathy or drug-related ILD/pneumonitis was reported.
A Phase 3 global study of SKB264/sac-TMT monotherapy versus standard of care (SOC) chemotherapy in 3L+ gastric/GEJ adenocarcinoma is being planned.
Press Contact: Xinwei Li, klbio_pr@kelun.com
2024-03-23
More

The World Meteorological Organization mentioned on the Interim Report on the State of the Global Climate 2023 that the year 2023 is the hottest year in the history of mankind since records have been kept, and the global climate is getting warmer and warmer, and the earth that we live on is facing a serious climate crisis, and energy conservation and environmental protection are imminent.

"Earth Hour was initiated by the World Wide Fund for Nature (WWF) in 2007, calling on people to voluntarily turn off their lights for one hour at the end of March each year to protect our planet.
Today, Earth Hour has become the world's largest national environmental event, focusing on the twin crises of nature loss and climate change on a global scale.
As a result of the global effort, by 2023, more than 410,000 hours have been accumulated in the "Hour Bank", an online counter for taking positive action to protect the planet.

Earth Hour 2024 will be held at 20:30pm local time on March 23, 2024, to inspire people in more than 190 countries and regions to take action to protect our common home.
Responding to the call of WWF, Kelun-Biotech actively participates in Earth Hour, and carries out all-round publicity for its employees by posting posters, broadcasting on electronic screens and sending out announcements, advocating employees to participate in the one hour lights out activity at home or in the office area from 20:30 to 21:30 on March 23rd, and encouraging employees to post Earth Hour related articles. At the same time, employees were encouraged to post Earth Hour related social media to call on their friends and relatives to participate in the activity.

Environmental protection is more than just an hour. Outside of the event period, Kelun-Biotech also actively fulfills its corporate social responsibility by practicing sustainable environmental protection and helping to move towards the environmental goal of zero load. For example, in 2023, we will save 680,000kWh of electricity by choosing energy-saving chiller units; save more than 240,000 standard cubic meters of natural gas by optimizing the air-fuel ratio of boilers; and save 35,000 tons of water by renovating some water points with recycled water.

We prefer larger or recyclable packaging, and in 2023, we will replace the outer packaging of 5L chemical reagents for R&D from cartons to plastic baskets, and replace dichloromethane and ethyl acetate from small drums to large iron drums, to effectively reduce waste emissions through control at the source.
We invite you to join us on March 23rd at 20:30 to gather the power of "One Hour", turn off unnecessary lighting, and use small actions to bring about a big change with the heart of fraternity, and build a naturally good and carbon-neutral future together.

About Kelun-Biotech
Kelun-Biotech(6990.HK)is a holding subsidiary of Kelun Pharmaceutical (002422.SZ), which focuses on the R&D, manufacturing, commercialization and global collaboration of innovative biological drugs and small molecule drugs. The company focuses on major disease areas such as solid tumors, autoimmune, inflammatory, and metabolic diseases, and in establishing a globalized drug development and industrialization platform to address the unmet medical needs in China and the rest of world. The Company is committed to becoming a leading global enterprise in the field of innovative drugs.
At present, the Company has more than 30 ongoing innovative projects in major disease areas such as solid tumors, autoimmune, inflammatory, and metabolic diseases, including over 10 projects in the clinical stage and 4 projects in the NDA stage with several global trials being conducted simultaneously in multiple countries, including China, Europe, and the United States. The company has established one of the world’s leading proprietary ADC platforms, OptiDC?, and has 5 ADC projects in the clinical stage (2 of which are in the NDA stage) and several projects in the preclinical stage. For more information, please visit https://kelun-biotech.com/.
2024-03-19
More

(“Kelun-Biotech” or the “Company”, SEHK: 6990.HK), an innovative TROP2-ADC (SKB264, MK-2870), a randomized, open, multicenter study to evaluate the efficacy of SKB264 in the first-line treatment of chemotherapy in patients with recurrent or metastatic non-resectable triple-negative breast cancer (TNBC) in advanced stages. SKB264 comparing investigator-selected chemotherapy for the first-line treatment of patients with non-surgically resectable recurrent or metastatic triple-negative breast cancer (TNBC) to a randomized, open, multicenter Phase III registry-based clinical study in patients with advanced-stage, non-systemic-treatment-na?ve PD-L1-negative TNBC (the “OptiTROP Breast03 (“OptiTROP Breast03” or “the Study”) was successfully held in Hefei.

This study a clinical trial evaluating SKB264 comparing investigator-selected chemotherapy first-line for the treatment of patients with non-operable resectable recurrent or metastatic triple-negative breast cancer (Protocol No. SKB264-III-11), with the national lead research center being the Affiliated Cancer Hospital of Fudan University, the lead researcher being Prof. Zhi-Min Shao, and the clinical registry numbers being: CTR20240413, NCT06279364, and is currently being rapidly pushed into the group.

Professor Zhimin Shao, Chairman of the conference, said, “It is a great honor to lead the registrational clinical study of ColumboTech SKB264 in triple negative breast cancer. Currently, TNBC is a hot area of clinical research. We believe that based on the excellent performance of TROP2 ADC SKB264 reported in first-line as well as second-line and above treatment of advanced TNBC in triple-negative breast cancer in the previous phase, SKB264 can achieve better clinical results in the first-line treatment of triple-negative breast cancer in China. We sincerely thank all the experts for their active participation in this trial, and hope that through our joint efforts and professional wisdom, we can make positive contributions to this clinical study, and look forward to SKB264 receiving marketing approval as soon as possible for the benefit of more patients”.

Dr. Ge Junyou shared, “Kelun is the world's largest manufacturer of infusion solutions in terms of production scale, the world's largest manufacturer of antibiotic intermediates in terms of production scale, and a leading head enterprise of pharmaceutical innovation in China. Kelun-Biotech carries the champion gene of the Group and adheres to innovation at source.SKB264 is the first TROP2 ADC-like drug in China to enter Phase III and enter NDA registration.SKB264's NDA filing for TNBC backline treatment has been accepted by the State Drug Administration, and the drug has been recognized as a breakthrough therapy by four organizations, and has initiated a number of clinical studies in different indications in addition to TNBC. The drug has also received four breakthrough therapeutic recognitions. We look forward to working together to rapidly advance the OptiTROP Breast03 study and accelerate the marketing process for this indication, which will benefit more breast cancer patients. Thank you for the great support from all the experts!”

Professor Cao Min from Hunan Cancer Hospital shared his detailed experience in terms of center overview and enrollment, case sharing, screening and enrollment process management, and internal and external multi-party collaboration.

Professor Liang Yan from Jiangsu Provincial People's Hospital shared detailed experience from center enrollment, case sharing, subjects in group management and management of common adverse events.

Professor Fan Lei from Fudan University Cancer Hospital gave a detailed presentation on the program design and implementation details of the project.

Under the chairmanship of Prof. Zhimin Shao and Dr. Xiaoping Jin, Chief Medical Officer of Clonbuterol, all the participating experts had a full and enthusiastic discussion on the study design, enrollment criteria of the study population, and the study process, and reached a consensus.

Professor Wang Zhonghua and Prof. Shao Zhimin from the Affiliated Cancer Hospital of Fudan University summarized the meeting at the end and thanked all the experts who participated in the investigator meeting. The investigators' meeting came to a successful conclusion, officially starting a brand-new journey for the OptiTROP Breast03 study. Clonbuterol has been exploring the advantageous areas of ADC technology in depth, and continues to promote the deepening layout of SKB264 clinical research. SKB264 first-line treatment of non-surgically resectable locally advanced, recurrent or metastatic PD-L1 TNBC has also been recognized by the BTD, and Clonbuterol will efficiently and qualitatively complete the registry study, and make joint efforts with the first-line clinical force to bring more hope and gospel to global tumor patients. Gospel.
About Kelun-Biotech:
Kelun-Biotech(6990.HK)is a holding subsidiary of Kelun Pharmaceutical (002422.SZ), which focuses on the R&D, manufacturing, commercialization and global collaboration of innovative biological drugs and small molecule drugs. The company focuses on major disease areas such as solid tumors, autoimmune, inflammatory, and metabolic diseases, and in establishing a globalized drug development and industrialization platform to address the unmet medical needs in China and the rest of world. The Company is committed to becoming a leading global enterprise in the field of innovative drugs.
At present, the Company has more than 30 ongoing innovative projects in major disease areas such as solid tumors, autoimmune, inflammatory, and metabolic diseases, including over 10 projects in the clinical stage and 4 projects in the NDA stage with several global trials being conducted simultaneously in multiple countries, including China, Europe, and the United States. The company has established one of the world’s leading proprietary ADC platforms, OptiDC?, and has 5 ADC projects in the clinical stage (2 of which are in the NDA stage) and several projects in the preclinical stage. For more information, please visit https://kelun-biotech.com/.
2024-03-14
More
Micro-world at your fingertips, customized nature :
“In this season of spring blossoming and grass growing, during the “Goddess Month” in March, the company issued holiday gifts and presents to all female employees to send holiday greetings and best wishes, and organized a March March Goddess Month handmade activities.

One bottle, one world, Kelun-Biotech’s micro landscape eco-bottle DIY activity took female employees to put the warmth of greenery into glass bottles, feel the power of plants together, and add a touch of fresh fun to life.
Crystal clear glass bottles, green moss plants, staggered landscape fabrics, fantasy cartoon dolls... In the fun atmosphere, everyone made the bright spring mood into unique limited edition works.

This handcraft activity not only stimulated everyone's curiosity about things in nature and knowledge of ferns and mosses, but also allowed everyone's creative thinking to be displayed in an exclusive space, conveying the concept of living with love for greenery and the environment.
Ancient style lantern DIY, enjoy the thousand years of cultural customs:
Lantern is one of the typical elements of China's traditional culture and a symbol of good luck. This month, Kelun-Biotech carried out the DIY activity of ancient style lanterns, leading the staff to appreciate the millennium culture and customs.

This activity is a traditional and modern fusion of cultural feast. Staff do-it-yourself, exquisite ancient style lanterns gradually presented in front of everyone, in the staff to exercise the ability to do it at the same time, but also harvested their own holiday exclusive gift, heritage and promote the excellent traditional culture of the Chinese nation, feel the beauty of the holiday and warmth, effectively enrich the cultural life of the staff, set up a platform for interaction, communication, mutual assistance.

About Kelun-Biotech: Kelun-Biotech(6990.HK)is a holding subsidiary of Kelun Pharmaceutical (002422.SZ), which focuses on the R&D, manufacturing, commercialization and global collaboration of innovative biological drugs and small molecule drugs. The company focuses on major disease areas such as solid tumors, autoimmune, inflammatory, and metabolic diseases, and in establishing a globalized drug development and industrialization platform to address the unmet medical needs in China and the rest of world. The Company is committed to becoming a leading global enterprise in the field of innovative drugs.
At present, the Company has more than 30 ongoing innovative projects in major disease areas such as solid tumors, autoimmune, inflammatory, and metabolic diseases, including over 10 projects in the clinical stage and 4 projects in the NDA stage with several global trials being conducted simultaneously in multiple countries, including China, Europe, and the United States. The company has established one of the world’s leading proprietary ADC platforms, OptiDC?, and has 5 ADC projects in the clinical stage (2 of which are in the NDA stage) and several projects in the preclinical stage. For more information, please visit https://kelun-biotech.com/.
2022年09月06日
我们决定向甘孜泸定、雅安石棉捐赠300万元现金、300万元物资,目前已成功对接甘孜州红十字会、雅安市红十字会,今天下午已经完成打款,物资根据当地所需正在紧急集结。对于灾区需要的其他支持,我们也当全力以赴。”
9月6日下午,四川科伦药业股份有限公司相关负责人告诉记者,针对四川泸定6.8级地震中受灾严重的泸定县和石棉县,他们紧急启动灾害应急处理方案,并进行现金和物资捐赠。