我们决定向甘孜泸定、雅安石棉捐赠300万元现金、300万元物资,目前已成功对接甘孜州红十字会、雅安市红十字会,今天下午已经完成打款,物资根据当地所需正在紧急集结。对于灾区需要的其他支持,我们也当全力以赴。”
9月6日下午,四川科伦药业股份有限公司相关负责人告诉记者,针对四川泸定6.8级地震中受灾严重的泸定县和石棉县,他们紧急启动灾害应急处理方案,并进行现金和物资捐赠。
2026-02-06
CHENGDU, China, February 4, 2026—Sichuan Kelun-Biotech Biopharmaceutical Co., Ltd. ("Kelun-Biotech" or the "Company", 6990.HK) announced that a new indication application for its TROP2-directed ADC sacituzumab tirumotecan (sac-TMT, also known as SKB264/MK-2870) (佳泰莱®) has been approved by the National Medical Products Administration (NMPA) of China for treatment of adult patients with unresectable or metastatic HR+/HER2- (IHC 0, IHC 1+ or IHC 2+/ISH-) breast cancer (BC) who have received prior endocrine therapy (ET) and at least one line of chemotherapy in advanced setting. This approval for HR+/HER2- BC after at least one prior line of chemotherapy marks the fourth indication for sac-TMT approved for marketing in China.
The approval is based on the positive results from the Phase III OptiTROP-Breast02 study which was selected as a Late-Breaking Abstract (LBA) and presented as an oral report at the 2025 European Society for Medical Oncology (ESMO) Congress.
The OptiTROP-Breast02 study evaluated the efficacy and safety of sac-TMT monotherapy compared to investigator's choice of chemotherapy in patients with unresectable or metastatic HR+/HER2- BC. Of the patients enrolled in this Phase III study, 95.7% had visceral metastases, 75.9% had liver metastases; 52.9% were HER2-zero (IHC 0), while 47.1% were HER2-low (IHC 1+ or IHC 2+/ISH-). All patients had received prior CDK4/6 inhibitor and taxane therapy; 56.6% had received ≥2 lines of prior chemotherapy in the advanced or metastatic setting.
Results showed that sac-TMT demonstrated a statistically significant and clinically meaningful increase in progression-free survival (PFS) as assessed by the Blinded Independent Central Review (BICR) compared to chemotherapy (8.3 vs. 4.1 months; hazard ratios (HR), 0.35; 95% CI: 0.26-0.48; p<0.0001). Consistent PFS benefits were observed across all pre-specified subgroups, including HER2-zero and HER2-low, number of chemotherapy lines received in the advanced or metastatic setting, presence of baseline visceral and liver metastases and previous CDK4/6 inhibitor use. According to BICR-assessed PFS results, the hazard ratios in the HER2-zero and HER2-low (IHC 1+ or IHC 2+/ISH-) subgroups were 0.39 (95% CI: 0.26-0.57) and 0.31 (95% CI: 0.20-0.48), respectively. A trend towards overall survival (OS) benefit and a significantly higher objective response rate (ORR) (41.5% vs. 24.1%) were also observed compared with chemotherapy. [1]
Currently, Phase III clinical studies of sac-TMT with or without pembrolizumab (KEYTRUDA®[2]) for the treatment of chemotherapy-naïve HR+/HER2- BC who have received prior ET have been initiated globally (NCT06312176) and in China (NCT07071337).
About HR+/HER2- Breast Cancer
Breast cancer is one of the most common malignant tumors that seriously threaten women's health worldwide. In 2022, there were about 2,297,000 new cases of breast cancer and 666,000 deaths worldwide. Among them, HR+/HER2- breast cancer is the most common subtype, accounting for about 70% of all breast cancer cases, and advanced HR+/HER2- breast cancer has a poor prognosis. This subtype is typically sensitive to hormonal therapy, and therefore, endocrine therapy combined with a CDK4/6 inhibitor constitutes the standard treatment. However, for patients with HR+/HER2- advanced breast cancer whose disease progresses on endocrine therapy, chemotherapy is widely used in clinical while it is associated with low response rate (ORR approximately 14%-22.9%) and limited survival benefit (mPFS approximately 4.0-4.9 months).
About Sac-TMT
Sac-TMT, a core product of the Company, is a novel human TROP2 ADC in which the Company has proprietary intellectual property rights, targeting advanced solid tumors such as non-small cell lung cancer (NSCLC), breast cancer (BC), gastric cancer (GC), gynecological tumors, among others. Sac-TMT is developed with a novel linker to conjugate the payload, a belotecan-derivative topoisomerase I inhibitor with a drug-to-antibody-ratio (DAR) of 7.4. Sac-TMT specifically recognizes TROP2 on the surface of tumor cells by recombinant anti-TROP2 humanized monoclonal antibodies, which is then endocytosed by tumor cells and releases the payload KL610023 intracellularly. KL610023, as a topoisomerase I inhibitor, induces DNA damage to tumor cells, which in turn leads to cell-cycle arrest and apoptosis. In addition, it also releases KL610023 in the tumor microenvironment. Given that KL610023 is membrane permeable, it can enable a bystander effect, or in other words kill adjacent tumor cells.
In May 2022, the Company licensed the exclusive rights to MSD (the tradename of Merck & Co., Inc, Rahway, NJ, USA) to develop, use, manufacture and commercialize sac-TMT in all territories outside of Greater China (which includes Mainland China, Hong Kong, Macao and Taiwan).
To date, four indications for sac-TMT have been approved and marketed in China for: EGFR mutant-positive locally advanced or metastatic non-squamous NSCLC following progression on EGFR-TKI therapy and platinum-based chemotherapy; unresectable locally advanced or metastatic TNBC who have received at least two prior systemic therapies (at least one of them for advanced or metastatic setting); EGFR mutant-positive locally advanced or metastatic non-squamous NSCLC who progressed after treatment with EGFR-TKI therapy; unresectable or metastatic HR+/HER2- (IHC 0, IHC 1+ or IHC 2+/ISH-) BC who have received prior ET and at least one line of chemotherapy in advanced setting. The first two indications listed above have been included in China's National Reimbursement Drug List (NRDL). This inclusion is expected to bring clinical benefits to a greater number of patients with BC and NSCLC. Additionally, sac-TMT has been granted six Breakthrough Therapy Designations (BTDs) by the NMPA.
Sac-TMT is the world's first TROP2 ADC drug approved for marketing in lung cancer. As of today, Kelun-Biotech has initiated 9 registrational clinical studies in China. MSD is evaluating16 ongoing Phase III global clinical studies of sac-TMT as a monotherapy or with pembrolizumab or other anti-cancer agents for several types of cancer. These studies are sponsored and led by MSD.
About Kelun-Biotech
Kelun-Biotech (6990.HK) is a holding subsidiary of Kelun Pharmaceutical, which focuses on the R&D, manufacturing, commercialization and global collaboration of innovative biological drugs and small molecule drugs. Kelun-Biotech focuses on major disease areas such as solid tumors, autoimmune, and metabolic diseases, and in establishing a globalized drug development and industrialization platform to address the unmet medical needs in China and the rest of world. Kelun-Biotech is committed to becoming a leading global enterprise in the field of innovative drugs. At present, Kelun-Biotech has more than 30 ongoing key innovative drug projects, of which 4 projects have been approved for marketing, more than 10 projects are in the clinical stage. Kelun-Biotech has established one of the world's leading proprietary ADC and novel DC platforms, OptiDC™, and has 2 ADC projects approved for marketing, and multiple ADC and novel DC assets in clinical or preclinical research stage. For more information, please visit https://en.kelun-biotech.com/.
Reference Sources:
[1] Fan Y, Li H, Wang H, et al. ESMO Congress 2025, LBA23.
[2] KEYTRUDA® (pembrolizumab) is a registered trademark of Merck Sharp & Dohme LLC (MSD), a subsidiary of Merck & Co., Inc., Rahway, NJ, USA.
2026-01-16
CHENGDU, China, Jan. 16, 2026 /PRNewswire/ -- From January 12 to 15, 2026, the 44th J.P. Morgan Healthcare Conference (JPMHC) was held in San Francisco, California, USA. Dr. Ge Michael, President and CEO of Sichuan Kelun-Biotech Biopharmaceutical Co., Ltd. ("Kelun-Biotech" or the "Company", 6990.HK), was invited to attend the conference and delivered a keynote speech on the morning of January 15 (local time) and presented the Company's latest achievements in drug R&D, commercialization, and globalization, and outlined its innovation strategy and future development plans.
Since initiating its innovation journey in 2012, Kelun-Biotech has rapidly emerged as a leader in China's innovative drug field, building differentiated technology platforms and robust R&D pipelines. Leveraging its global leading OptiDC™ platform for antibody-drug conjugates (ADCs) and novel drug conjugates (DCs), the Company continuously advances the differentiated development of ADCs and novel DCs, forming a gradient portfolio for treating multiple tumor types. Currently, Kelun-Biotech has two ADC products on market: sacituzumab tirumotecan (sac-TMT, 佳泰莱®) and trastuzumab botidotin (舒泰莱®), covering breast cancer and lung cancer indications. Additionally, nine uniquely designed ADC and novel DC drugs—including cutting-edge directions such as bispecific ADC and radiopharmaceutical conjugate (RDC) are in clinical stage. For high-incidence tumor types in China, such as breast cancer, lung cancer, and gastrointestinal tumors, the Company has initiated nine pivotal studies, while multiple Phase II clinical studies targeting gynecological tumors are progressing steadily. Furthermore, Kelun-Biotech has also developed several non-DC candidates and expanded indications into non-oncology areas.
Over the past year, Kelun-Biotech has presented multiple research findings at international academic conferences and published in authoritative journals: three were selected for oral presentations at the American Society of Clinical Oncology (ASCO) annual meeting, and three were selected as Late-Breaking Abstracts (LBA) for oral presentations at the European Society for Medical Oncology (ESMO) Congress. Among these, the results of the OptiTROP-Lung04 study of sac-TMT for treating EGFR-mutant Non-Small Cell Lung Cancer (NSCLC) after TKI therapy were presented at the Presidential Symposium session of ESMO and simultaneously published in The New England Journal of Medicine, highlighting its global academic and clinical value.
In terms of commercialization, Kelun-Biotech has formed a competitive initial product portfolio. Its core product, the TROP2-directed ADC sac-TMT, has been approved in China for three indications: second-line and above triple-negative breast cancer, second-line and third-line EGFR-mutated NSCLC. The HER2-directed ADC trastuzumab botidotin was approved last year for second-line and above HER2-positive breast cancer, becoming the first domestically developed HER2-directed ADC approved for this indication. Furthermore, the anti-EGFR monoclonal antibody Cetuximab N01 (达泰莱®) for RAS wild-type colorectal cancer and the anti-PD-L1 monoclonal antibody tagitanlimab (科泰莱®) for nasopharyngeal carcinoma have been launched. Another small-molecule RET inhibitor A400 is expected to be approved within the year, bringing the total number of commercialized products in China to five. Currently, three of the Company's commercialized products, covering five indications, have been included in the National Reimbursement Drug List (NRDL), further benefiting a broad population of cancer patients.
While strengthening its presence in the domestic market, Kelun-Biotech is actively expanding overseas. It has established collaborations with MSD, Ellipses, Windward Bio and Crescent Biopharma to maximize the value of its pipeline value and corporate worth. Among these, MSD is evaluating 16 global Phase III clinical studies of sac-TMT.
The breakthroughs in both clinical development and commercialization are driven by the Company's sustained investment in innovative R&D. With over a decade of accumulation in the ADC field, Kelun-Biotech's proprietary OptiDC™ platform enables differentiated design of drug candidates, which combines specific targets or targeting mechanisms with the most suitable payload-linker strategy to balance efficacy and safety. Additionally, the company is adopting a "multi-pronged" innovation strategy to continuously enhance platform capabilities. By exploring novel targets, new payloads, and diverse conjugation technologies, it is expanding the application boundaries across both oncology and non-oncology fields.
Looking ahead, Kelun-Biotech will consolidate its foundations in R&D, technologies, platforms, and operations by Executing five key development strategies. Concurrently, the Company will elevate its globalization strategy, enhancing its capabilities in product development, registration, and commercialization in ex-China market, advancing on its path to becoming a world-class biopharmaceutical company.
Please refer to the "Investor Relations-Investor Calendar" page of the company's official website for the presentation materials. You can visit the website for further details.
About Kelun-Biotech
Kelun-Biotech (6990.HK) is a holding subsidiary of Kelun Pharmaceutical (002422.SZ), which focuses on the R&D, manufacturing, commercialization and global collaboration of innovative biological drugs and small molecule drugs. Kelun-Biotech focuses on major disease areas such as solid tumors, autoimmune, and metabolic diseases, and in establishing a globalized drug development and industrialization platform to address the unmet medical needs in China and the rest of world. Kelun-Biotech is committed to becoming a leading global enterprise in the field of innovative drugs. At present, Kelun-Biotech has more than 30 ongoing key innovative drug projects, of which 4 projects have been approved for marketing, 1 project is in the NDA stage and more than 10 projects are in the clinical stage. Kelun-Biotech has established one of the world's leading proprietary ADC and novel DC platforms, OptiDC™, and has 2 ADC projects approved for marketing, and multiple ADC and novel DC assets in clinical or preclinical research stage. For more information, please visit https://en.kelun-biotech.com/.
2026-01-07
The 44th J.P. Morgan Healthcare Conference (JPMHC) will be held in San Francisco, California, USA, from January 12 to 15, 2026. Sichuan Kelun-Biotech Biopharmaceutical Co., Ltd. (“Kelun-Biotech” or the “Company”, 6990.HK) has been invited to attend the conference and Dr. Michael Ge, President and CEO of Kelun-Biotech, will share the company’s latest business progresses as well as its innovation capabilities and strategies.
Presentation Time: Thursday, January 15, 2026 at 9:30 AM Pacific Standard Time
Presentation Venue: Westin St. Francis, San Francisco, USA
J.P. Morgan Annual Healthcare Conference is honored as a “benchmark for development and investment in the global healthcare area”. During the conference, Kelun-Biotech will join massive seasoned experts and industry leaders to discuss cutting-edge trends in biopharmaceutical innovation. Based on the ongoing transformation moment of the global pharmaceutical industry, the company will actively pursue mutually beneficial partnership opportunities on the international stage.
You can visit “Investor Relations - Investor Calendar” page of Kelun-Biotech’s official website for participation of live webcast of presentation session, and watch replay within 30 days after the conference.
About Kelun-Biotech
Kelun-Biotech (6990.HK) is a holding subsidiary of Kelun Pharmaceutical (002422.SZ), which focuses on the R&D, manufacturing, commercialization and global collaboration of innovative biological drugs and small molecule drugs. Kelun-Biotech focuses on major disease areas such as solid tumors, autoimmune, and metabolic diseases, and in establishing a globalized drug development and industrialization platform to address the unmet medical needs in China and the rest of world. Kelun-Biotech is committed to becoming a leading global enterprise in the field of innovative drugs. At present, Kelun-Biotech has more than 30 ongoing key innovative drug projects, of which 4 projects have been approved for marketing, 1 project is in the NDA stage and more than 10 projects are in the clinical stage. Kelun-Biotech has established one of the world's leading proprietary ADC and novel DC platforms, OptiDC™, and has 2 ADC projects approved for marketing, and multiple ADC and novel DC assets in clinical or preclinical research stage. For more information, please visit https://en.kelun-biotech.com/.
2026-01-05
CHENGDU, China, Jan. 5, 2026 /PRNewswire/ -- Sichuan Kelun-Biotech Biopharmaceutical Co., Ltd. ("Kelun-Biotech" or the "Company", 6990.HK) announced that its Investigational New Drug (IND) application for SKB105 (also known as CR-003), an internally developed integrin beta-6 (ITGB6)-targeted antibody-drug conjugate (ADC), has been approved by the Center for Drug Evaluation (CDE) of the National Medical Products Administration (NMPA) of China for the treatment of advanced solid tumors.
In December 2025, Kelun-Biotech and Crescent Biopharma, Inc. ("Crescent") entered into a strategic collaboration for SKB105/CR-003 and SKB118 (a PD-1 x VEGF bispecific antibody, also known as CR-001). Under the collaboration, Kelun-Biotech granted Crescent exclusive rights to research, develop, manufacture and commercialize SKB105/CR-003 in the United States, Europe and all other markets outside of Greater China. In addition, Crescent granted Kelun-Biotech exclusive rights to research, develop, manufacture and commercialize SKB118/CR-001 in Greater China. The IND application for SKB118/CR-001 has been cleared by the U.S. Food and Drug Administration (FDA) with a global Phase I/II clinical trial for the treatment of advanced solid tumors is set to commence shortly. Kelun-Biotech plans to submit an IND application for SKB118/CR-001 to the Center for Drug Evaluation of the National Medical Products Administration of China in the near future.
About SKB105 (also known as CR-003)
SKB105 is a differentiated ADC targeting ITGB6 with a topoisomerase I inhibitor payload. ITGB6 is overexpressed in many solid tumors, but shows minimal to no expression in most normal tissues, thereby potentially reducing the risk of systemic toxicity and off-target effects. SKB105 incorporates proprietary Kthiol® irreversible conjugation technology, linking an anti-ITGB6 fully human Immunoglobulin G1 (IgG1) monoclonal antibody (mAb) to a clinically validated cleavable linker. This design aims to enhance stability and tumor-specific payload delivery while reducing adverse effects. In preclinical models, SKB105 demonstrated a favorable efficacy, safety, and pharmacokinetic (PK) profile.
About SKB118(also known as CR-001)
SKB118/CR-001 is a tetravalent bispecific antibody being developed for the treatment of solid tumors that combines two complementary, validated mechanisms in oncology via a blockade of PD-1 and VEGF. PD-1 checkpoint inhibition is aimed at restoring T cells' ability to recognize and destroy tumor cells, and blocking VEGF is intended for reducing blood supply to tumor cells and inhibiting tumor growth. In preclinical studies, SKB118/CR-001 demonstrated cooperative pharmacology with increased binding to PD-1 and signal blockade in the presence of VEGF as well as robust anti-tumor activity. SKB118/CR-001's anti-VEGF activity may also normalize the vasculature at the tumor site, which has the potential to improve the localization and effectiveness of combination therapies, particularly in conjunction with ADCs.
About Kelun-Biotech
Kelun-Biotech (6990.HK) is a holding subsidiary of Kelun Pharmaceutical (002422.SZ), which focuses on the R&D, manufacturing, commercialization and global collaboration of innovative biological drugs and small molecule drugs. Kelun-Biotech focuses on major disease areas such as solid tumors, autoimmune, inflammatory, and metabolic diseases, and in establishing a globalized drug development and industrialization platform to address the unmet medical needs in China and the rest of world. Kelun-Biotech is committed to becoming a leading global enterprise in the field of innovative drugs. At present, Kelun-Biotech has more than 30 ongoing key innovative drug projects, of which 4 projects have been approved for marketing, 1 project is in the NDA stage and more than 10 projects are in the clinical stage. Kelun-Biotech has established one of the world's leading proprietary ADC and novel DC platforms, OptiDC™, and has 2 ADC projects approved for marketing, and multiple ADC and novel DC assets in clinical or preclinical research stage. For more information, please visit https://en.kelun-biotech.com/.
2026-01-05
CHENGDU, China, January 5, 2026 -- Sichuan Kelun-Biotech Biopharmaceutical Co., Ltd. (“Kelun-Biotech” or the “Company,” 6990.HK) today announced that its TROP2-directed antibody-drug conjugate (ADC) sacituzumab tirumotecan (sac-TMT, also known as SKB264/MK-2870) (佳泰莱®) in combination with MSD’s anti-PD-1 monoclonal antibody pembrolizumab (KEYTRUDA®[1]) was granted Breakthrough Therapy Designation (BTD) by the Center for Drug Evaluation (CDE) of the National Medical Products Administration (NMPA) of China for the first-line treatment of patients with locally advanced or metastatic non-small cell lung cancer (NSCLC) who have PD-L1 tumor proportion score (TPS)≥1% and are EGFR-negative and ALK-negative.
BTD is granted for treatment regimens that provide effective treatment or prevention for conditions with no currently available therapy, or that demonstrate significant clinical advantages over currently available treatments. For drugs included in the breakthrough therapy process, if relevant conditions are met, applications for conditional approval and priority review and approval can be submitted when applying for marketing authorization.
Previously, the company announced that results from the Phase III clinical trial of OptiTROP-Lung05, evaluating sac-TMT in combination with pembrolizumab as first-line treatment for PD-L1-positive NSCLC, demonstrated a statistically significant and clinically meaningful improvement in its primary endpoint of progression-free survival (PFS). A positive trend was also observed in overall survival (OS). OptiTROP-Lung05 is the first Phase III study of an immunotherapy and ADC combination to meet its primary endpoint in the first-line treatment of NSCLC. Granting of BTD for the first-line treatment of PD-L1-positive NSCLC indication offers pathways to expedite the review and potential approval process of sac-TMT for this indication.
To date, sac-TMT has received five BTDs for:
locally advanced or metastatic triple-negative breast cancer (TNBC) in July 2022;
locally advanced or metastatic EGFR-mutant NSCLC after progression on EGFR-TKI therapy in January 2023;
locally advanced or metastatic hormone-receptor positive (HR+) and human epidermal growth factor receptor 2-negative (HER2-) breast cancer (BC) in patients who have previously received at least two lines of systemic chemotherapy in June 2023;
first-line treatment of unresectable locally advanced, recurrent or metastatic PD-L1 negative TNBC in March 2024;
In combination with anti-PD-L1 monoclonal antibody tagitanlimab for the first-line treatment of locally advanced or metastatic non-squamous NSCLC without actionable genomic alterations in June 2025.
About sac-TMT
Sac-TMT, a core product of the Company, is a novel human TROP2 ADC in which the Company has proprietary intellectual property rights, targeting advanced solid tumors such as NSCLC, BC, gastric cancer (GC), gynecological tumors, among others. Sac-TMT is developed with a novel linker to conjugate the payload, a belotecan-derivative topoisomerase I inhibitor with a drug-to-antibody-ratio (DAR) of 7.4. Sac-TMT specifically recognizes TROP2 on the surface of tumor cells by recombinant anti-TROP2 humanized monoclonal antibodies, which is then endocytosed by tumor cells and releases the payload KL610023 intracellularly. KL610023, as a topoisomerase I inhibitor, induces DNA damage to tumor cells, which in turn leads to cell-cycle arrest and apoptosis. In addition, it also releases KL610023 in the tumor microenvironment. Given that KL610023 is membrane permeable, it can enable a bystander effect, or in other words kill adjacent tumor cells.
In May 2022, the Company licensed the exclusive rights to MSD (the tradename of Merck & Co., Inc, Rahway, NJ, USA) to develop, use, manufacture and commercialize sac-TMT in all territories outside of Greater China (which includes Mainland China, Hong Kong, Macao and Taiwan).
To date, three indications for sac-TMT have been approved and marketed in China for: EGFR mutant-positive locally advanced or metastatic non-squamous NSCLC following progression on EGFR-TKI therapy and platinum-based chemotherapy; Unresectable locally advanced or metastatic TNBC who have received at least two prior systemic therapies (at least one of them for advanced or metastatic setting); EGFR mutant-positive locally advanced or metastatic non-squamous NSCLC who progressed after treatment with EGFR-TKI therapy. The first two indications listed above have been included in China's National Reimbursement Drug List (NRDL). This inclusion is expected to bring clinical benefits to a greater number of cancer patients.
Sac-TMT is the world's first TROP2 ADC drug approved for marketing in lung cancer. In addition, the sNDA for sac-TMT for second-line and above treatment of HR+/HER2- BC was accepted by the Center for Drug Evaluation of the National Medical Products Administration, and was included in the priority review and approval process.
As of today, Kelun-Biotech has initiated 9 registrational clinical studies in China. MSD has initiated 15 ongoing Phase 3 global clinical studies of sac-TMT as a monotherapy or with pembrolizumab or other anti-cancer agents for several types of cancer. These studies are sponsored and led by MSD.
About Kelun-Biotech
Kelun-Biotech (6990.HK) is a holding subsidiary of Kelun Pharmaceutical (002422.SZ), which focuses on the R&D, manufacturing, commercialization and global collaboration of innovative biological drugs and small molecule drugs. Kelun-Biotech focuses on major disease areas such as solid tumors, autoimmune, inflammatory, and metabolic diseases, and in establishing a globalized drug development and industrialization platform to address the unmet medical needs in China and the rest of world. Kelun-Biotech is committed to becoming a leading global enterprise in the field of innovative drugs. At present, Kelun-Biotech has more than 30 ongoing key innovative drug projects, of which 4 projects have been approved for marketing, 1 project is in the NDA stage and more than 10 projects are in the clinical stage. Kelun-Biotech has established one of the world's leading proprietary ADC and novel DC platforms, OptiDC™, and has 2 ADC projects approved for marketing, and multiple ADC and novel DC assets in clinical or preclinical research stage. For more information, please visit https://en.kelun-biotech.com/.
[1] KEYTRUDA® (pembrolizumab) is a registered trademark of Merck Sharp & Dohme LLC (MSD), a subsidiary of Merck & Co., Inc., Rahway, NJ, USA.
2025-12-04
Companies to advance CR-001, a PD-1 x VEGF bispecific antibody, and SKB105, an integrin beta-6-directed antibody-drug conjugate (ADC), in global markets and China
Collaboration designed to accelerate and expand the development of synergistic combinations with CR-001 and ADCs, including SKB105
CR-001 and SKB105 on track to enter Phase 1/2 monotherapy clinical trials in Q1 2026 with combination studies to follow
CHENGDU, China and WALTHAM, Mass., Dec. 4, 2025 /PRNewswire/ -- Sichuan Kelun-Biotech Biopharmaceutical Co., Ltd. ("Kelun-Biotech", 6990.HK), which focuses on the R&D, manufacturing, commercialization and global collaboration of innovative biological drugs and small molecule drugs, and Crescent Biopharma, Inc. ("Crescent") (Nasdaq: CBIO), a biotechnology company dedicated to rapidly advancing the next wave of therapies for cancer patients, today announced that the companies have entered into a strategic partnership to develop and commercialize oncology therapeutics, including novel combinations.
The partnership involves Crescent's CR-001, a PD-1 x VEGF bispecific antibody, and Kelun-Biotech's SKB105, an integrin beta-6 (ITGB6)-directed antibody-drug conjugate (ADC) with a topoisomerase payload. Both candidates are being developed for the treatment of solid tumors and are expected to enter Phase 1/2 monotherapy clinical trials in the first quarter of 2026.
Under the terms of the collaboration, Crescent has granted Kelun-Biotech exclusive rights to research, develop, manufacture and commercialize CR-001 in Greater China (including mainland China, Hong Kong, Macau and Taiwan). In addition, Kelun-Biotech has granted Crescent exclusive rights to research, develop, manufacture and commercialize SKB105 in the United States, Europe and all other markets outside of Greater China. The partnership includes the development of these candidates as monotherapies, and also the evaluation of CR-001 in combination with SKB105.Both Crescent and Kelun-Biotech have the right to independently develop CR-001 in additional combinations, including combinations of CR-001 with proprietary ADC pipeline assets.
Dr. Michael Ge, chief Executive officer of Kelun-Biotech, said, "We are pleased to have entered into a partnership with Crescent for two innovative assets, CR-001 and SKB105. This collaboration complements and strengthens our differentiated oncology pipeline by the addition of CR-001 and also enables us to advance the development of SKB105 in the global market, bolstering its potential commercial value and our global collaboration network. Our creative global partnership combines the capabilities of both companies to explore novel monotherapies and combination strategies for tumor treatments with SKB105 and CR-001. By leveraging China's abundant clinical resources and Execution efficiency, we aim to expedite clinical development while rigorously maintaining the highest global standards. We believe this partnership creates a powerful synergy to maximize the potential of these two drug candidates for the treatment of patients in both China and the rest of the world."
"We are thrilled to be partnering with Kelun-Biotech, an established leader in the development and commercialization of ADCs who shares our commitment to bringing next generation therapeutics that can improve outcomes for people living with cancer," said Joshua Brumm, chief Executive officer of Crescent. "This collaboration expands our pipeline with the addition of SKB105, furthers our strategy of advancing multiple modalities across our portfolio, and accelerates our efforts to deliver synergistic combinations with CR-001, which has the potential to be a foundational backbone therapy. We look forward to working with Kelun-Biotech to drive innovative therapeutics with the potential to address multiple tumor types and transform cancer care."
Under the collaboration, Kelun-Biotech will receive an upfront payment of US$80 million from Crescent and is also eligible to receive additional milestones of up to US$1.25 billion, plus tiered middle single-digit to low double-digit royalties on net sales of SKB105. Kelun-Biotech is also eligible to receive additional payment from Crescent if Crescent undergoes a near-term change of control or enters into a sublicense agreement with a third party. Crescent will receive an upfront payment of US$20 million from Kelun-Biotech and is also eligible to receive additional milestones of up to US$30 million, plus tiered low to middle single digit royalties on net sales of CR-001.
About CR-001 (also known as SKB118)
CR-001 is a tetravalent bispecific antibody being developed for the treatment of solid tumors that combines two complementary, validated mechanisms in oncology via a blockade of PD-1 and VEGF. PD-1 checkpoint inhibition is aimed at restoring T cells' ability to recognize and destroy tumor cells, and blocking VEGF is intended for reducing blood supply to tumor cells and inhibiting tumor growth. In preclinical studies, CR-001 demonstrated cooperative pharmacology with increased binding to PD-1 and signal blockade in the presence of VEGF as well as robust anti-tumor activity. CR-001's anti-VEGF activity may also normalize the vasculature at the tumor site, which has the potential to improve the localization and effectiveness of combination therapies, such as the administration of CR-001 with antibody-drug conjugates (ADCs). A global Phase 1/2 trial of CR-001 in patients with solid tumors is anticipated to commence in the first quarter of 2026.
About SKB105 (also known as CR-003)
SKB105 is a differentiated ADC targeting integrin beta-6 (ITGB6) with a topoisomerase 1 inhibitor payload. ITGB6 is overexpressed in many solid tumors, but shows minimal to no expression in most normal tissues, thereby potentially reducing the risk of systemic toxicity and off-target effects. SKB105 consists of an anti-ITGB6 fully human IgG1 monoclonal antibody conjugated via a stable, clinically validated cleavable linker. The molecule incorporates proprietary Kthiol® irreversible conjugation technology, designed to enhance stability and tumor-specific payload delivery while reducing adverse effects. SKB105 demonstrated a favorable efficacy, safety, and pharmacokinetic (PK) profile in preclinical models. A Phase 1/2 clinical trial of SKB105 in patients with solid tumors is anticipated to commence in the first quarter of 2026.
About Kelun-Biotech
Kelun-Biotech (6990.HK) is a holding subsidiary of Kelun Pharmaceutical (002422.SZ), which focuses on the R&D, manufacturing, commercialization and global collaboration of innovative biological drugs and small molecule drugs. Kelun-Biotech focuses on major disease areas such as solid tumors, autoimmune, inflammatory, and metabolic diseases, and in establishing a globalized drug development and industrialization platform to address the unmet medical needs in China and the rest of world. Kelun-Biotech is committed to becoming a leading global enterprise in the field of innovative drugs. At present, Kelun-Biotech has more than 30 ongoing key innovative drug projects, of which 4 projects have been approved for marketing, 1 project is in the NDA stage and more than 10 projects are in the clinical stage. Kelun-Biotech has established one of the world's leading proprietary ADC and novel DC platforms, OptiDC™, and has 2 ADC projects approved for marketing, and multiple ADC and novel DC assets in clinical or preclinical research stage. For more information, please visit https://en.kelun-biotech.com/.
About Crescent Biopharma
Crescent Biopharma's vision is to build a world leading oncology company bringing the next wave of therapies for cancer patients. Crescent 's pipeline includes its lead program, a PD-1 x VEGF bispecific antibody, as well as novel antibody-drug conjugates (ADCs). By leveraging multiple modalities and established targets, Crescent aims to rapidly advance potentially transformative therapies either as single agents or as part of combination regimens to treat a range of solid tumors. For more information, visit www.crescentbiopharma.com and follow Crescent on LinkedIn and X.
2025-10-20
After a median follow-up of 18.9 months, the median PFS was 8.3 months in the sac-TMT group and 4.3 months in the chemotherapy group (hazard ratio (HR), 0.49; 95% confidence interval (CI), 0.39 to 0.62; two-sided P<0.0001).
OS was significantly longer with sac-TMT than with chemotherapy (HR, 0.60; 95% CI, 0.44 to 0.82; two-sided P=0.001); 18-month OS rate was 65.8% and 48.0%, respectively. In the supplementary analysis, in which data were censored at the start date of subsequent ADC, the HR was 0.56 (95% CI, 0.41 to 0.77).
Sac-TMT was associated with a higher incidence of stomatitis with most cases being mild and with grade 3 or higher cases reported in very few patients (4.8%; all were grade 3). Low incidence of ocular-surface toxic effects, no ILD or pneumonitis, and one infusion-related reaction (grade 2) was reported in the sac-TMT group.
On October 20, results from the Phase III registrational clinical study OptiTROP-Lung04—led by Professor Zhang Li's team from Sun Yat-sen University Cancer Center—were published in the New England Journal of Medicine (Impact Factor = 78.5). The study evaluated the efficacy and safety of the TROP2 antibody-drug conjugate (ADC) sacituzumab tirumotecan (sac-TMT) monotherapy versus pemetrexed plus platinum chemotherapy for the treatment of patients with epidermal growth factor receptor (EGFR)-mutant locally advanced or metastatic non-squamous non-small cell lung cancer (NSCLC) who have progressed failed after treatment with EGFR-tyrosine kinase inhibitor (TKI) therapy. The study was also selected as a Late-Breaking Abstract (LBA) at the 2025 European Society for Medical Oncology (ESMO) Congress and was presented as an oral report in the Presidential Symposium (Presentation # LBA5).
OptiTROP-Lung04 is a randomized, open-label, multicenter Phase III study designed to evaluate the efficacy and safety of sac-TMT monotherapy versus pemetrexed plus platinum in patients with EGFR-mutated locally advanced or metastatic NSCLC who had progressed on prior EGFR-TKI therapy. Eligible patients were those with histologically and/or cytologically confirmed disease. The primary endpoint was Progression-Free Survival (PFS) assessed by a Blinded Independent Review Committee (BIRC) per RECIST v1.1, and the key secondary endpoint was overall survival (OS). Results showed that compared with platinum-based doublet chemotherapy, sac-TMT monotherapy demonstrated a statistically significant and clinically meaningful improvement in both PFS and OS. Consistent PFS and OS benefits were observed across all prespecified subgroups, including prior EGFR-TKI therapy, presence of liver or brain metastases, and EGFR mutation subtype.
Based on the positive results of the OptiTROP-Lung04 study, sac-TMT has been approved by the National Medical Products Administration (NMPA) in China for the treatment of adult patients with EGFR mutation-positive locally advanced or metastatic non-squamous NSCLC who have progressed after EGFR-TKI therapy. To date, sac-TMT monotherapy remains the only approved treatment option for advanced EGFR-mutant NSCLC patients who have progressed after prior TKI therapy, as well as those who have progressed after prior TKI and platinum-based chemotherapy (either concurrently or sequentially). This achievement provides comprehensive coverage for the entire population of TKI-resistant patients.
Dr. Michael Ge, Chief Executive Officer of Kelun-Biotech, commented: “OptiTROP-Lung04 is a key study that propels sac-TMT from late-line to an earlier-line treatment-setting in lung cancer, achieving a significant improvement in overall survival. We are thrilled that the results have been published in The New England Journal of Medicine, allowing this important finding to be shared widely among medical professionals and providing a reference for future lung cancer research. Together with our partner MSD, we will continue to advance sac-TMT’s clinical development and regulatory approvals, covering broader lung cancer and other indications, with the goal of making sac-TMT a cornerstone therapy for the benefit of patients.”
Professor Shengxiang Ren from the Department of Oncology at Shanghai Pulmonary Hospital, Tongji University, stated: “The application of third-generation EGFR-TKIs has significantly improved the overall prognosis for patients with advanced EGFR-mutant NSCLC. However, drug resistance is almost inevitable. Traditional treatment after resistance involves platinum-based doublet chemotherapy, which offers limited overall benefit. In recent years, regimens of chemotherapy plus immune checkpoint inhibitors+anti-angiogenic agents, or EGFR/c-Met bsAb, or PD-1/VEGF bsAb, have been successively approved for this indication. However, all these approaches are chemotherapy-based, highlighting an urgent need to explore novel treatment regimens. The OptiTROP-Lung04 study confirms sac-TMT as the first treatment option as a monotherapy to deliver clear dual benefits in both PFS and OS following EGFR-TKI progression. The sequential application of sac-TMT prior to chemotherapy demonstrates strong competence, establishing itself as a new standard of care for patients with advanced EGFR-mutant lung cancer after TKI resistance.”
About Sac-TMT
Sac-TMT, a core product of the Company, is a novel human TROP2 ADC in which the Company has proprietary intellectual property rights, targeting advanced solid tumors such as NSCLC, breast cancer (BC), gastric cancer (GC), gynecological tumors, among others. Sac-TMT is developed with a novel linker to conjugate the payload, a belotecan-derivative topoisomerase I inhibitor with a drug-to-antibody-ratio (DAR) of 7.4. Sac-TMT specifically recognizes TROP2 on the surface of tumor cells by recombinant anti-TROP2 humanized monoclonal antibodies, which is then endocytosed by tumor cells and releases the payload KL610023 intracellularly. KL610023, as a topoisomerase I inhibitor, induces DNA damage to tumor cells, which in turn leads to cell-cycle arrest and apoptosis. In addition, it also releases KL610023 in the tumor microenvironment. Given that KL610023 is membrane permeable, it can enable a bystander effect, or in other words kill adjacent tumor cells.
In May 2022, the Company licensed the exclusive rights to MSD (the tradename of Merck & Co., Inc., Rahway, NJ, USA) to develop, use, manufacture and commercialize sac-TMT in all territories outside of Greater China (includes Mainland China, Hong Kong, Macau, and Taiwan).
To date, three indications for sac-TMT have been approved and marketed in China for the treatment of adult patients with unresectable locally advanced or metastatic triple negative breast cancer (TNBC) who have received at least two prior systemic therapies (at least one of them for advanced or metastatic setting), EGFR mutation-positive locally advanced or metastatic non-squamous NSCLC following progression on EGFR-TKI therapy and platinum-based chemotherapy and EGFR mutant-positive locally advanced or metastatic non-squamous NSCLC who progressed after treatment with EGFR-TKI therapy. Sac-TMT is the first TROP2 ADC drug approved for marketing in lung cancer globally. In addition, the new indication application for sac-TMT for the treatment of adult patients with unresectable locally advanced, metastatic hormone receptor positive (HR+) and human epidermal growth factor receptor 2-negative (HER2-) BC who have received prior endocrine therapy and other systemic treatments in the advanced or metastatic setting was accepted by the Center for Drug Evaluation of the NMPA, and was included in the priority review and approval process.
As of today, the Company has initiated 9 registrational clinical studies in China. MSD has initiated 15 ongoing Phase Ⅲ global clinical studies of sac-TMT as a monotherapy or with pembrolizumab1 or other anti-cancer agents for several types of cancer. These studies are sponsored and led by MSD.
About Kelun-Biotech
Kelun-Biotech (6990.HK) is a holding subsidiary of Kelun Pharmaceutical (002422.SZ), which focuses on the R&D, manufacturing, commercialization and global collaboration of innovative biological drugs and small molecule drugs. The company focuses on major disease areas such as solid tumors, autoimmune, inflammatory, and metabolic diseases, and in establishing a globalized drug development and industrialization platform to address the unmet medical needs in China and the rest of world. The Company is committed to becoming a leading global enterprise in the field of innovative drugs. At present, the Company has more than 30 ongoing key innovative drug projects, of which 4 projects have been approved for marketing, 1 project is in the NDA stage and more than 10 projects are in the clinical stage. The company has established one of the world's leading proprietary ADC and novel DC platforms, OptiDC™, and has 2 ADC projects approved for marketing, and multiple ADC and novel DC assets in clinical or preclinical research stage. For more information, please visit https://kelun-biotech.com/.
References:
1. Pembrolizumab (KEYTRUDA®) is a registered trademark of Merck Sharp & Dohme LLC (MSD), a subsidiary of Merck & Co., Inc., Rahway, NJ, USA.
2025-10-19
CHENGDU, China, Oct, 18 , 2025——Sichuan Kelun-Biotech Biopharmaceutical Co., Ltd. (the “Company”) announced that at the 2025 European Society for Medical Oncology (ESMO) Congress held in Berlin, Germany, Results from a Phase 3 study of the Company’s human epidermal growth factor receptor 2 (HER2)-directed ADC trastuzumab botidotin (also known as A166) trastuzumab botidotin versus trastuzumab emtansine (T-DM1) in HER2-positive unresectable or metastatic BC was presented as an oral report by Professor Xichun Hu from Fudan University Shanghai Cancer Center (Presentation # LBA24, Proffered paper session 1: Breast cancer, metastatic).
Trastuzumab botidotin is a HER2-targeted ADC composed of a cytotoxic drug (Duostatin-5, anti-microtubule agent) with site-specific conjugation to trastuzumab via a stable protease-cleavable valine-citrulline linker. The unique linker is stable in plasma and selectively cleaved by lysosomal cathepsin B that is up-regulated in cancer cells.
In this study, a total of 365 patients with HER2+ unresectable or metastatic BC who had received at least one prior anti-HER2 therapy were randomized (1:1) to receive trastuzumab botidotin or T-DM1. 53% of patients had received ≥2 lines of anti-HER2 therapy, 61% of patients had HER2 Immunohistochemistry (IHC) 3+, and 60% of patients had been treated with TKIs, particularly pyrotinib (56%). As of April 26, 2025, median follow-up was 14.9 months.
Median PFS was significantly longer in trastuzumab botidotin than in T-DM1 (11.1 months vs 4.4 months; HR: 0.39, 95% CI, 0.30-0.51, p<0.0001). PFS benefit with trastuzumab botidotin was consistently observed regardless of prior lines of anti-HER2 therapy (HR 0.36, 95% CI, 0.25-0.53, for 1 prior line; HR 0.39, 95% CI, 0.28-0.56, for ≥2 prior lines).
ORR by blinded independent central review (BICR) was 76.9% vs 53.0%.
A trend toward benefit in OS was observed in trastuzumab botidotin (HR 0.62; 95% CI, 0.38-1.03).
Grade ≥3 treatment emergent adverse events (TEAEs) occurred in 69.8% of patients in trastuzumab botidotin and 63.7% in T-DM1. The most common TEAEs associated with dose reduction were ocular AEs for trastuzumab botidotin, and were platelet count decreased for trastuzumab emtansine. Only two patients permanently discontinued trastuzumab botidotin due to TEAE. No on-treatment deaths were observed in trastuzumab botidotin, compared with 1.6% in T-DM1, all of which were considered unrelated to treatment.
As a conclusion, this second head-to-head trial comparing T-DM1 with other anti-HER2 regimens demonstrated that trastuzumab botidotin statistically improved PFS with an ORR of 76.9% vs 53.0%. PFS benefit with trastuzumab botidotin was consistently observed regardless of prior lines of anti-HER2 therapy. Ocular AEs were also manageable.
"Professor Xichun Hu, National Lead Principal Investigator from Fudan University Shanghai Cancer Center:“Trastuzumab botidotin effectively balances safety and efficacy through its unique molecular design, reducing the incidence of interstitial lung disease and hematologic toxicity. According to research data, trastuzumab botidotin demonstrated significant survival benefits in the pivotal Phase III trial, with an overall manageable safety profile, providing a new important treatment option for pretreated HER2+ BC patients. These positive results also offer robust evidence-based support for personalized treatment and updates to clinical practice guidelines.”
About Trastuzumab botidotin
Trastuzumab botidotin is a differentiated HER2 ADC to treat advanced HER2+ solid tumors. As an innovative HER2 ADC developed by the Company, it conjugates a novel, monomethyl auristatin F (MMAF) derivative (a highly cytotoxic tubulin inhibitor, Duo-5) via a stable, enzyme-cleavable linker to a HER2 monoclonal antibody with a DAR of 2. Trastuzumab botidotin specifically binds to HER2 on the surface of tumor cells and is internalized by tumor cells, releasing the toxin molecule Duo-5 inside the cell. Duo-5 induces tumor cell cycle arrest in the G2/M phase, leading to tumor cell apoptosis. After targeting HER2, trastuzumab botidotin can also inhibit the HER2 signaling pathway; it has antibody-dependent cell-mediated cytotoxicity (ADCC) activity.
Based on the results of a multi-center, randomized, open-label, controlled, Phase 3 KL166-III-06 study, trastuzumab botidotin was approved for marketing by the NMPA in for adult patients with unresectable or metastatic HER2 positive BC who have received one or more prior anti-HER2 therapy. At a pre-specified interim analysis, trastuzumab botidotin demonstrated a statistically significant and clinically meaningful improvement in the primary endpoint of PFS as assessed by the BICR compared with T-DM1[; the beneficial trend for OS of trastuzumab botidotin was also observed.
Currently, the Company has initiated an open, multi-center Phase 2 clinical study of trastuzumab botidotin in the treatment of HER2+ unresectable or metastatic BC that previously received a topoisomerase inhibitor ADC.
About Kelun-Biotech
Kelun-Biotech (6990.HK) is a holding subsidiary of Kelun Pharmaceutical (002422.SZ), which focuses on the R&D, manufacturing, commercialization and global collaboration of innovative biological drugs and small molecule drugs. The company focuses on major disease areas such as solid tumors, autoimmune, inflammatory, and metabolic diseases, and in establishing a globalized drug development and industrialization platform to address the unmet medical needs in China and the rest of world. The Company is committed to becoming a leading global enterprise in the field of innovative drugs. At present, the Company has more than 30 ongoing key innovative drug projects, of which 4 projects have been approved for marketing, 1 projects are in the NDA stage and more than 10 projects are in the clinical stage. The company has established one of the world's leading proprietary ADC and novel DC platforms, OptiDC™, and has 1 ADC project approved for marketing and multiple ADC and novel DC assets in clinical or preclinical research stage. For more information, please visit https://kelun-biotech.com/.
2025-10-18
CHENGDU, China, Oct, 19, 2025——Sichuan Kelun-Biotech Biopharmaceutical Co., Ltd. (the “Company”) announced that at the 2025 European Society for Medical Oncology (ESMO) Congress held in Berlin, Germany, results from a Phase 3 OptiTROP-Lung04 trial of the Company’s trophoblast cell-surface antigen 2 (TROP2)-directed antibody-drug conjugate (ADC) sacituzumab tirumotecan (sac-TMT) in EGFR-mutated non-small cell lung cancer (NSCLC) following progression on epidermal growth factor receptor-tyrosine kinase inhibitors (EGFR-TKIs) was presented as an oral report by Professor Li Zhang from Sun Yat-sen University Cancer Center (Presentation # LBA5, Presidential Symposium II) and were simultaneously published in the New England Journal of Medicine (Impact Factor = 78.5).
In the OptiTROP-Lung04 trial, a total of 376 patients were randomized (1:1) to receive sac-TMT monotherapy or chemotherapy.
As at the data cut-off date July 06, 2025, the median follow-up is 18.9 months. the median Progression-Free Survival (PFS) was 8.3 months in the sac-TMT group and 4.3 months in the chemotherapy group. Sac-TMT significantly improved PFS over chemotherapy with 51% lower risk of disease progression or death (hazard ratio (HR) 0.49; 95% confidence interval (CI), 0.39-0.62; P<0.0001).
At the preplanned interim analysis of overall survival (OS), the OS was not reached (NR) in the sac-TMT group and 17.4 months in the chemotherapy group. sac-TMT significantly improved OS over chemotherapy with 40% lower risk of death (hazard ratio (HR) 0.6; 95% CI: 0.44-0.82; two-sided P=0.001). In the supplemental analysis, when censoring patients at the date of initiation of subsequent ADCs, sac-TMT significantly improved OS over chemotherapy with 44% lower risk of death (HR, 0.56; 95% CI, 0.41 - 0.77).
Sac-TMT significantly improved ORR as compared to chemotherapy (60.6% vs 43.1%)
A consistent PFS and OS benefit of sac-TMT over chemotherapy was observed across all predefined subgroups, including prior EGFR-TKI therapy, presence of liver or brain metastases, and EGFR mutation subtype.
The incidence of any grade treatment-related adverse events (TRAEs) and grade ≥3 TRAEs was similar between the two groups. The most common TRAEs for both sac-TMT and chemotherapy were hematologic toxicities. No TRAEs led to discontinuation or death, and no cases of interstitial lung disease/pneumonitis were reported in the sac-TMT group. Ocular surface toxicity: occurred in 9.6% of patients in the sac-TMT group, all of which were grade 1 - 2.
As a conclusion, sac-TMT demonstrates highly statistically significant and clinically meaningful improvements in PFS and OS compared to platinum-based chemotherapy and showed a manageable safety profile, with no unexpected safety signals identified. Several global phase 3 studies of sac-TMT monotherapy (NCT06305754, NCT06074588) and combination study with osimertinib in China (NCT06670196) in EGFR-mutant NSCLC are ongoing.
Professor Zhang Li, National Lead Principal Investigator from Sun Yat-sen University Cancer Center, commented: "Compared to platinum-based doublet chemotherapy, sac-TMT not only significantly prolonged PFS but also demonstrated a statistically significant and clinically meaningful improvement in OS within this patient population. This achievement marks a major breakthrough in global lung cancer treatment—sac-TMT, as a monotherapy, demonstrated statistically significant and clinically meaningful improvements in both PFS and OS in the Phase III trial for patients with EGFR-TKI-resistant NSCLC. This study provides highly valuable, new evidence-based guidance for lung cancer management worldwide and has the potential to reshape the therapeutic landscape for EGFR-TKI-resistant NSCLC "
About sac-TMT
Sac-TMT, a core product of the Company, is a novel human TROP2 ADC in which the Company has proprietary intellectual property rights, targeting advanced solid tumors such as NSCLC, BC, GC, gynecological tumors, among others. Sac-TMT is developed with a novel linker to conjugate the payload, a belotecan-derivative topoisomerase I inhibitor with a drug-to-antibody-ratio (DAR) of 7.4. Sac-TMT specifically recognizes TROP2 on the surface of tumor cells by recombinant anti-TROP2 humanized monoclonal antibodies, which is then endocytosed by tumor cells and releases the payload KL610023 intracellularly. KL610023, as a topoisomerase I inhibitor, induces DNA damage to tumor cells, which in turn leads to cell-cycle arrest and apoptosis. In addition, it also releases KL610023 in the tumor microenvironment. Given that KL610023 is membrane permeable, it can enable a bystander effect, or in other words kill adjacent tumor cells.
In May 2022, the Company licensed the exclusive rights to MSD (the tradename of Merck & Co., Inc, Rahway, NJ, USA) to develop, use, manufacture and commercialize sac-TMT in all territories outside of Greater China (which includes Mainland China, Hong Kong, Macao and Taiwan).
To date, three indications for sac-TMT have been approved and marketed in China for the treatment of adult patients with unresectable locally advanced or metastatic triple negative breast cancer (TNBC) who have received at least two prior systemic therapies (at least one of them for advanced or metastatic setting), EGFR mutation-positive locally advanced or metastatic non-squamous NSCLC following progression on EGFR-TKI therapy and platinum-based chemotherapy and EGFR mutant-positive locally advanced or metastatic non-squamous NSCLC who progressed after treatment with EGFR-TKI therapy. Sac-TMT is the first TROP2 ADC drug approved for marketing in lung cancer globally. In addition, the new indication applications for sac-TMT for the treatment of adult patients with unresectable locally advanced, metastatic HR+/HER2- BC who have received prior endocrine therapy and other systemic treatments in the advanced or metastatic setting was accepted by the Center for Drug Evaluation (CDE) of the National Medical Products Administration (NMPA), and was included in the priority review and approval process.
As of today, the Company has initiated 9 registrational clinical studies in China. MSD has initiated 15 ongoing Phase 3 global clinical studies of sac-TMT as a monotherapy or with pembrolizumab or other anti-cancer agents for several types of cancer. These studies are sponsored and led by MSD.
About Kelun-Biotech
Kelun-Biotech (6990.HK) is a holding subsidiary of Kelun Pharmaceutical (002422.SZ), which focuses on the R&D, manufacturing, commercialization and global collaboration of innovative biological drugs and small molecule drugs. The company focuses on major disease areas such as solid tumors, autoimmune, inflammatory, and metabolic diseases, and in establishing a globalized drug development and industrialization platform to address the unmet medical needs in China and the rest of world. The Company is committed to becoming a leading global enterprise in the field of innovative drugs. At present, the Company has more than 30 ongoing key innovative drug projects, of which 4 projects have been approved for marketing, 1 project is in the NDA stage and more than 10 projects are in the clinical stage. The company has established one of the world's leading proprietary ADC and novel DC platforms, OptiDC™, and has 2 ADC project approved for marketing and multiple ADC and novel DC assets in clinical or preclinical research stage. For more information, please visit https://kelun-biotech.com/.
2024-01-11
More
The 42nd J.P. Morgan Annual Healthcare Conference(“JPMHC”)2024 is taking place on January 8-11 at San Fracisco, USA, members of Sichuan Kelun-Biotech Biopharmaceutical Co., Ltd. (6990.HK, Kelun-Biotech) senior management team participated in the conference. Dr. Micheal Ge, CEO of Kelun-Biotech, delivered a presentation about innovation strength and business progress of Kelun-Biotech.
Presentation Title: Dedicated to human health with a caring heart
Presentation Time: Pacific Standard Time (PST) Tuesday 8:00 AM, January 9, 2024

As one of the pioneers in the antibody-drug conjugates (ADC) development company, Kelun-Biotech has established world leading ADC platform “OptiDC?”, as well as a rich and differentiated ADC R&D pipeline covers unmet clinical needs in the field of oncology. Our core product Trops-ADC and Her2 ADC are expected to be approved for NDA in China this year. Meanwhile, Kelun-Biotech is accelerating the development progress of over 10 clinical and pre-clinical ADC and ADC-derivative products. With the support of the Kelun Group, Kelun-Biotech has built mature R&D, manufacturing and quality control system, the company is now building a commercialization team to form the marketing system. Kelun-Biotech’s innovation capability has gained high recognition from global partners, including MSD, and the capital market. Kelun-Biotech is rapidly developing a unique growth curve as an ADC R&D pharmaceutical enterprise.
Dr.Michael Ge said: “It’s our pleasure to meet again with global industry leaders, innovative technology creators and members of the investment community here at JPMHC. Our top priority is to rapidly advance our differentiated pipeline programs into and through the clinic for disease conditions with significant medical needs. We will continue to innovate and optimize our ADC platform, discover novel payloads, linkers, engineer novel ADC designs, and expand clinical application of antibody drug conjugates to non-oncology disease areas. Build upon our end-to-end drug development capabilities, infrastructure, and commercialization readiness. Furthermore, we plan to extend our global footprints through strategic partnerships and maximize our pipeline and portfolio value. We look forward to achieving and updating you on the 2024 milestones in the coming year”.
Presentation Slides is available on the investor relations page of Kelun-Biotech website at: 投资者关系 - 四川科伦博泰生物医药股份有限公司 (kelun-biotech.com)
About Kelun-Biotech
Kelun-Biotech(6990.HK)is a holding subsidiary of Kelun Pharmaceutical (002422.SZ), which focuses on the R&D, manufacturing, commercialization and global collaboration of innovative biological drugs and small molecule drugs. The company focuses on major disease areas such as solid tumors, autoimmune, inflammatory, and metabolic diseases, and in establishing a globalized drug development and industrialization platform to address the unmet medical needs in China and the rest of world. The Company is committed to becoming a leading global enterprise in the field of innovative drugs. At present, the Company has 33 ongoing innovative projects in major disease areas such as solid tumors, autoimmune, inflammatory, and metabolic diseases, including 14 projects in the clinical stage with several global trials being conducted simultaneously in multiple countries, including China, Europe, and the United States. The company has established one of the world’s leading proprietary ADC platforms, OptiDC, and has four ADC projects in the clinical stage (two of which are in the NDA stage) and several projects in the preclinical stage. For more information, please visit https://kelun-biotech.com/.
2024-01-01
More
Sequence of time change, the Chinese chapter is new, on the occasion of this wonderful moment of resignation and welcome the new year, Kelun-Biotech to the hard work and dedication of all the staff and their families, to care for and support the development of the company from all walks of life of friends, to send the most sincere greetings and the best wishes!
Looking back on the past year, we have jointly experienced the turbulence and test of the industry, the company showed strong will and extraordinary perseverance to overcome the difficulties and achieved remarkable results. From product research and development to commercialization capacity building, every step condenses the sweat and wisdom of all colleagues.
During the year, we adhered to the innovation-driven development strategy and actively explored new ways of cutting-edge technologies and treatments for major diseases. By strengthening our biology and translational medicine research capabilities, we enhanced the efficiency and success rate of innovative drug development, and several innovative projects were awarded preclinical candidate compounds and one IND application was approved. Based on the advantages of our existing technology platform, we accelerated the research and development of new ADC drugs, launched the layout of radionuclide coupled drugs (RDC), and continued to expand the new direction of drug coupling technology, so as to solidify the foundation for the continuous enhancement of the value of our innovation pipeline.
During the year, we insisted on concentrating our efforts on advancing the clinical research progress of our projects, and continued to explore and rapidly validate the clinical value of our core projects, and the marketing applications of three products, A166 (Sutera?, HER2-ADC), A140 (Dattelite?, cetuximab), and SKB264 (Jatelle?, MK-2870, TROP2-ADC), were approved by the National Drug Administration's Center for Drug Evaluation (CDE). The Center for Drug Evaluation (CDE) of the State Drug Administration accepted the registration of three products, six new studies were approved by the CDE for clinical trials, and eight registered clinical studies were in full speed.
During the year, we insisted on striving for excellence, accelerated the construction of the industrial park, and continuously improved the quality of production. We established a life-cycle drug R&D and production quality system in line with international standards such as FDA, EU regulations and ICH guidelines, and continued to consolidate the integrated and comprehensive production platform. 2023 saw the completion of process validation of two projects, pre-production studies of three projects, NDA filing of three projects, and two-in-one inspection of registration and GMP of one project.
During the year, we insisted on deepening international cooperation and continued to optimize the alliance management process. We established a comprehensive technical communication system with Merck Sharp & Dohme to promote in-depth cooperation. We innovated the idea of cooperation, continuously explored new opportunities for project introduction and transfer, actively integrated into the global pharmaceutical industry chain, and fought our way forward from the global innovative drug-following development to the leading development.
This year, we launched the commercialization capacity building. We have fully absorbed industry talents to form a commercialization team with strong competitiveness; while carefully planning and formulating the promotion plan for our innovative products, we have actively expanded and enhanced the market influence and brand reputation of Clonbuterol and its innovative products, so as to lay a solid foundation for the future commercialization at a high level.
During the year, we seized the opportunities and pushed the company towards a broader development platform. on July 11, 2023, Kelun-Biotech was successfully listed on the Hong Kong Stock Exchange, which became the largest IPO in the healthcare sector in Hong Kong's capital market since 2022; it won the Best IPO Award of Hong Kong, China, by FinanceAsia 2023; and it won the Most Innovative Value Award of the Year in the list of the Most Valuable Leaders in Capital Markets of the Financial Times in 2023, and the Most Innovative Value Award of the Year. In the list of the Most Valuable Leaders in Capital Markets by Caixun in 2023, the company was honored with the Most Innovative Value Award of the Year, marking a major milestone in the innovation-driven development strategy of Coren.
All the achievements were made thanks to the strong leadership of the Board of Directors and the strong support of the Group, as well as the hard work of all colleagues. Hereby, we would like to express our highest respect and most sincere gratitude to all of you!
The yearly calendar has turned over a brand new page, and we are standing on a new starting line, with a glorious prospect unfolding in front of us. The roc rises with the wind one day, and rises ninety thousand miles. Let's work together to draw a new blueprint for Kolon in the pharmaceutical industry, continue to promote the construction of a healthy China, and contribute China's power to the global health of mankind.
In the new year, I wish you all a happy new year, a happy family and all your wishes!
January 1, 2024
2023-12-11
More
Sichuan Kelun-Biotech Biopharmaceutical Co., Ltd. (“Kelun-Biotech”, 6990.HK) announced that the new drug application (the “NDA”) for SKB264 (MK-2870, Brand name: 佳泰莱) in adult patients with unrespectable locally advanced, metastatic triple-negative breast cancer (TNBC) who have received at least two prior systemic therapies (at least one of them for advanced or metastatic setting) was accepted by the Center for Drug Evaluation (CDE) of the National Medical Products Administration (NMPA). SKB264 (MK-2870) is expected to be the first domestic TROP2-ADC to be approved for marketing in China. This SKB264 New Drug Application is based on a multi-center, randomized, controlled phase 3 clinical study of SKB264 (MK-2870) monotherapy for second line or above locally advanced or metastatic
SKB264 new drug application has been included in the priority review and approval process by the Center for Drug Evaluation (CDE) of the National Medical Products Administration (NMPA). For drugs that included in the priority review, CDE will prioritize the review and verification procedures to effectively shorten the approval timeframe for launching clinical-valuable new drugs to the market. The Company plans to publish the Phase III clinical trial data at the Academic Conference 2024 and promote the early launch of SKB264, to enrich the clinical treatments for patients with second-line and above TNBC.
Breast cancer is the number one malignant tumor that seriously threatens women's health all over the world, and its incidence is increasing year by year. TNBC is a subtype of breast cancer with specific molecular expression characteristics, invasive behavior and metastatic patterns, and has a poorer prognosis than other subtypes of breast cancer, with higher rates of local recurrence and distant metastasis, and the 5-year survival rate is only 13% for advanced TNBC patients (National Cancer Institute 2022). Due to the lack of therapeutic targets such as endocrine and HER2, TNBC is insensitive to both hormonal and targeted therapies, and the current treatment is still based on chemotherapeutic agents. For patients with second-line and above TNBC, the progression-free survival of current clinically available chemotherapy regimens is less than 3 months, and overall survival is about 5-8 months (Kazmi et al. 2020, O'Shaughnessy et al. 2021), and there is a large and urgent unmet clinical need for more effective therapeutic agents
On December 2023, Kelun-Biotech updated efficacy and safety results from a phase II expansion cohort in patients with previously treated metastatic TNBC for SKB264 (MK-2870) have been presented at the 46th San Antonio Breast Cancer Symposium (SABCS). (SABCS 2023 news link here)
About SKB264 (MK-2870)
SKB264 is a representative innovative ADC targeting TROP2, developed by Kelun-Biotech 's internationally renowned ADC research and development platform—OptiDC. It consists of a humanized anti-TROP2 monoclonal antibody with high affinity and targeting, combined with the self-developed small toxin molecule T030 (a topoisomerase I inhibitor) through a stability-optimized CL2A linker. The drug-to-antibody ratio (DAR) averages as high as 7.4. The stability of SKB264 has been enhanced in two main ways, allowing more ADC to effectively reach tumor cells. Firstly, the antibody end of the linker uses a sulfonamide pyrimidine connector, which allows for irreversible coupling with the antibody. Secondly, T030 contains a methyl sulfone structure, which can securely bind with the toxin end of the linker. The linker of SKB264 is affected by both extracellular pH-sensitive cleavage and intracellular enzymatic cleavage within tumor cells, which leads to efficient release of the small toxin molecule to exert its anti-tumor effects. T030 activity is similar to that of DXd and also shows a “bystander effect”. In summary, SKB264 exerts its anti-tumor effect in three ways. Firstly, the pH-sensitive linker can cleave and release T030 in the acidic tumor microenvironment. Secondly, after being engulfed by tumor cells, SKB264 releases T030 via enzymatic cleavage. Thirdly, T030 can also penetrate the cell membrane to exert a "bystander effect" to kill surrounding tumor cells. With a high-affinity monoclonal antibody, highly active toxin molecule, good stability, and high DAR value, the overall design of SKB264 contributes to its robust anti-tumor activity which has been preliminarily demonstrated in clinical studies to date.
In May 2022, the Company licensed the exclusive rights to MSD (the tradename of Merck & Co., Inc, Rahway, NJ, USA) to develop, use, manufacture and commercialize SKB264 (MK-2870) in all territories outside of Greater China (includes Mainland China, Hong Kong, Macao, and Taiwan).
SKB264 has received 3 BTDs from the CDE of China’s NMPA for the treatment of locally advanced or metastatic TNBC, locally advanced or metastatic EGFR-mutated non-small cell lung cancer which has failed EGFR-TKI therapy, and locally advanced or metastatic hormone receptor-positive (HR+) and human epidermal growth factor receptor 2-negative (HER2-) breast cancer which has received at least second-line systemic therapy.
About Kelun-Biotech
Kelun-Biotech (6990.HK) is a holding subsidiary of Kelun Pharmaceutical (002422.SZ), which focuses on the R&D, manufacturing, commercialization and global collaboration of innovative biological drugs and small molecule drugs. The company focuses on major disease areas such as solid tumors, autoimmune, inflammatory, and metabolic diseases, and in establishing a globalized drug development and industrialization platform to address the unmet medical needs in China and the rest of world. The Company is committed to becoming a leading global enterprise in the field of innovative drugs.
At present, the Company has 33 ongoing innovative projects in major disease areas such as solid tumors, autoimmune, inflammatory, and metabolic diseases, including 14 projects in the clinical stage with several global trials being conducted simultaneously in multiple countries, including China, Europe, and the United States. The company has established one of the world’s leading proprietary ADC platforms, OptiDC, and has four ADC projects in the clinical stage (two of which are in the Phase III or NDA stage) and several projects in the preclinical stage. For more information, please visit https://kelun-biotech.com/.
References:
1. National Cancer Institute (2022) Cancer Stat Facts: Female Breast Cancer Subtypes.
2.[Kazmi S, Chatterjee D, Raju D, et al. (2020)] Overall survival analysis in patients with metastatic breast cancer and liver or lung metastases treated with eribulin, gemcitabine, or capecitabine. Breast Cancer Research and Treatment; 184(2):559-565.
3.[O'Shaughnessy J, Punie K, Oliveira M, et al. (2021)] Assessment of sacituzumab govitecan (SG) versus treatment of physician’s choice (TPC) cohort by agent in the phase 3 ASCENT study of patients (pts) with metastatic triple-negative breast cancer (mTNBC). (2021): 1077-1077. https://meetinglibrary.asco.org/record/198258/abstract
Forward-Looking Statements
This press release contains certain forward-looking statements. These statements are based on the beliefs of our management as well as assumptions made by and information currently available to our management. When used in this press release, the words “aim,” “anticipate,” “believe,” “could,” “estimate,” “expect,” “going forward,” “intend,” “may,” “might,” “ought to,” “plan,” “potential,” “predict,” “project,” “seek,” “should,” “will,” “would” and the negative forms of these words and other similar expressions are intended to identify forward-looking statements. Such statements reflect the current views of our management with respect to future events, operations, liquidity and capital resources, some of which may not materialize or may change.
It is advised not to place any undue reliance on any forward-looking statements contained herein. The Company can give no assurance that these forward-looking statements will prove to have been correct. Expectations reflected in these forward-looking statements are subject to change and the Company undertakes no obligation to update or revise any forward-looking statements herein.
2023-12-07
More
The 46th American San Antonio Breast Cancer Symposium (SABCS) will be held from December 5th to 9th, Central Standard Time (CST), and the latest clinical results evaluating Kelun-Biotech's TROP2 ADC SKB264 (also known as MK-2870) will be presented in the form of a poster spotlight presentationthe Phase II expansion study for patients with locally advanced or metastatic triple-negative breast cancer (TNBC).
Previously, Kelun-Biotech had presented efficacy and safety data of SKB264 from a Phase II expansion study in TNBC at 2022 SABCS, and this year we will present updated results including overall survival (OS) data.
The Phase II expansion study includes 59 patients with previously treated locally advanced or metastatic TNBC who received SKB264 monotherapy, with a median follow-up time of 22.8 months (data cutoff date: May 5, 2023).
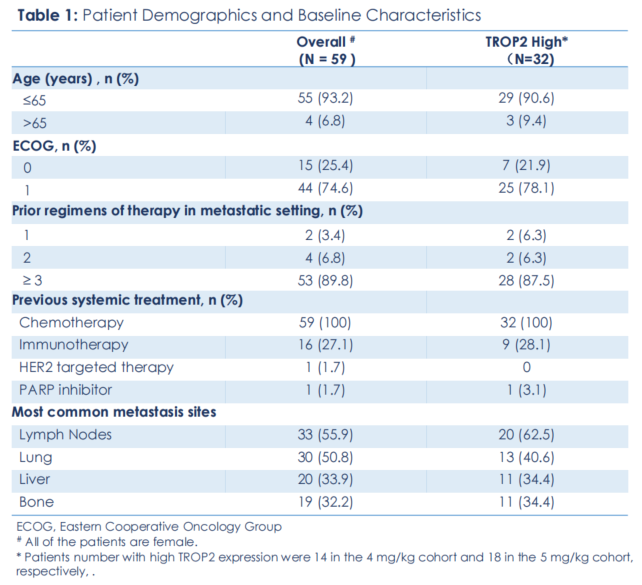
Baseline characteristics:
Among the 59 enrolled patients, 89.8% (53 patients) had previously received three or more treatment regimens, 27.1% (16 patients) had previously received immunotherapy, about half had lung metastases, and one-third had liver metastases.
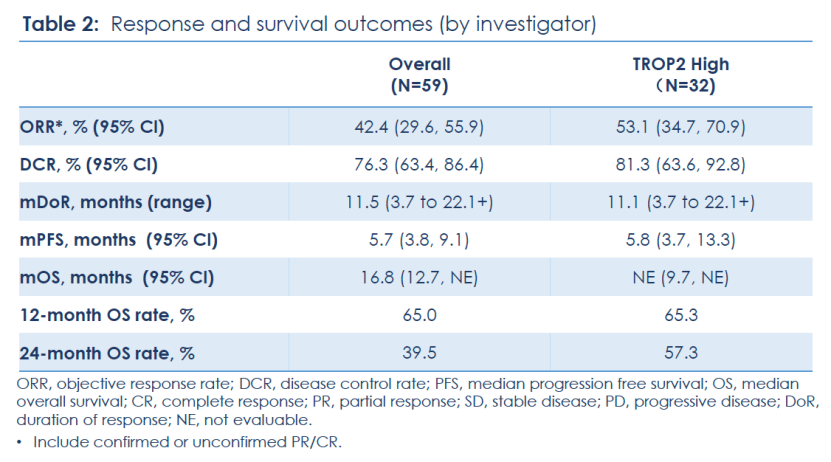
Efficacy:
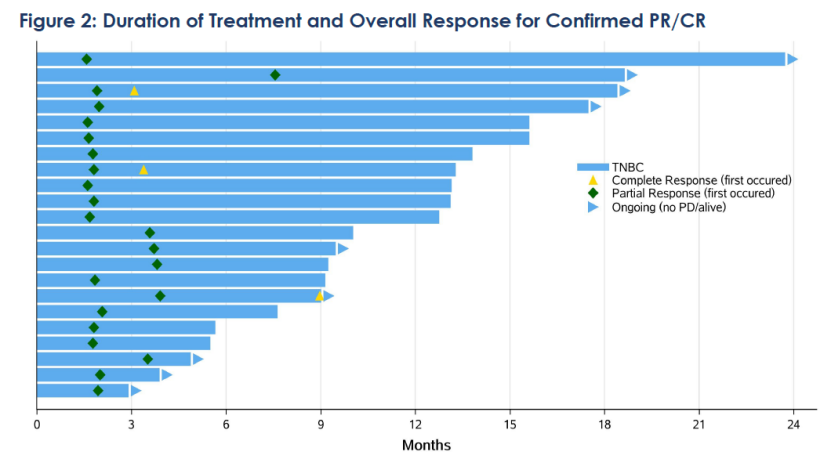
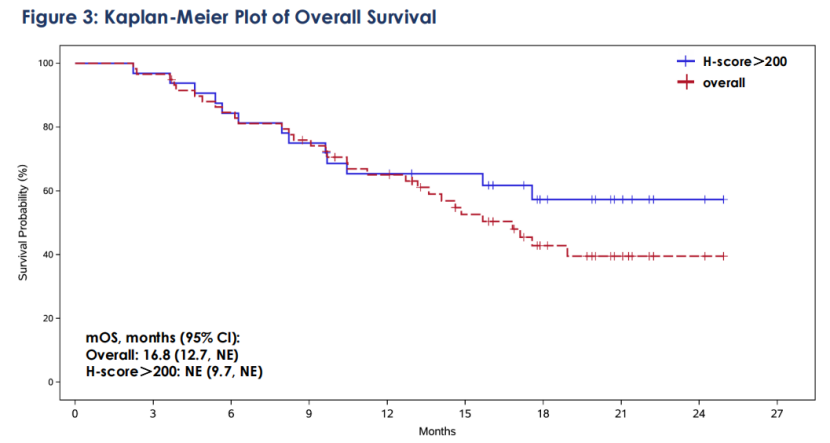
Among the 59 treated patients with locally advanced or metastatic TNBC, the objective response rate (ORR) was 42.4%, with 3 patients achieving complete response (CR); the disease control rate (DCR) was 76.3%; the median duration of response (mDoR) was 11.5 months. In addition, median progression-free survival (mPFS) was 5.7 months, and the median overall survival (mOS) reached 16.8 months, with 12-month and 24-month OS rates of 65% and 39.5%, respectively. Nearly 40% of patients in the study treated with SKB264 were alive at two years.. In the subgroup of patients with high TROP2 expression (H-score>200, n=32), the ORR was 53.1%, mDoR was 11.1 months, mPFS was 5.8 months, mOS had not been reached, and the 12-month and 24-month OS rates were 65.3% and 57.3% respectively.
Safety:
Treatment-related adverse events (TRAEs) were mainly hematologic toxicity, which was clinically manageable. The most common treatment-related adverse events (TRAEs ≥10%) of Grade 3 or higher were neutrophil count decrease, white blood cell count decrease, anemia, and platelet count decrease. TRAEs led to dose reduction in 13.6% of patients. No interstitial lung disease (ILD) or diarrhea of Grade 3 or higher was observed. No deaths occurred due to TRAE.
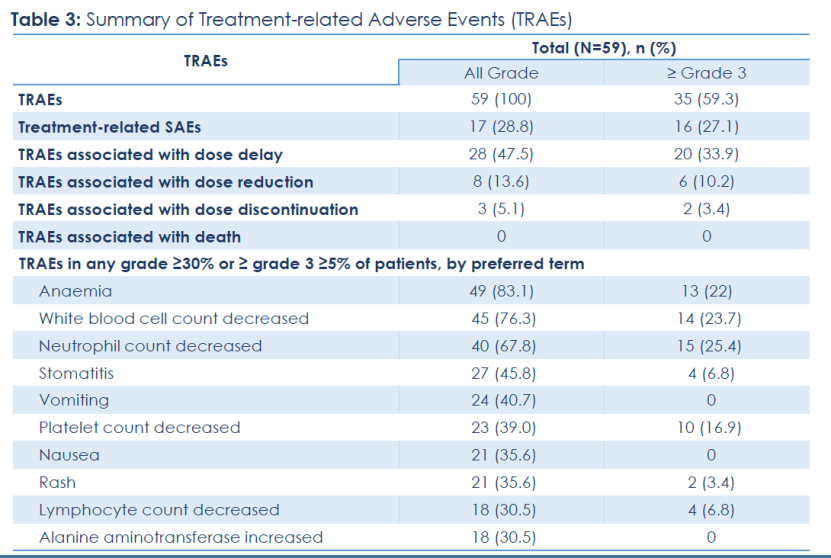
The updated clinical data indicated that patients with locally advanced or metastatic TNBC who have received multiple prior treatments may achieve sustained responses and extended overall survival (OS) trends with SKB264 treatment, and with manageable safety. Notably, a better response trend was observed in patients with locally advanced or metastatic TNBC with higher TROP2 expression.
Currently, the Phase III registrational clinical study of SKB264 monotherapy in at least second-line locally advanced or metastatic TNBC has reached the primary endpoint of improvement in PFS and has been included for priority review by the CDE. It is expected that a m product will be launched as soon as possible to benefit more patients.
About SKB264 (MK-2870)
SKB264 is a representative innovative ADC targeting TROP2, developed by Kelun-Biotech 's internationally renowned ADC research and development platform—OptiDC. It consists of a humanized anti-TROP2 monoclonal antibody with high affinity and targeting, combined with the self-developed small toxin molecule T030 (a topoisomerase I inhibitor) through a stability-optimized CL2A linker. The drug-to-antibody ratio (DAR) averages as high as 7.4. The stability of SKB264 has been enhanced in two main ways, allowing more ADC to effectively reach tumor cells. Firstly, the antibody end of the linker uses a sulfonamide pyrimidine connector, which allows for irreversible coupling with the antibody. Secondly, T030 contains a methyl sulfone structure, which can securely bind with the toxin end of the linker. The linker of SKB264 is affected by both extracellular pH-sensitive cleavage and intracellular enzymatic cleavage within tumor cells, which leads to efficient release of the small toxin molecule to exert its anti-tumor effects. T030 activity is similar to that of DXd and also shows a “bystander effect”. In summary, SKB264 exerts its anti-tumor effect in three ways. Firstly, the pH-sensitive linker can cleave and release T030 in the acidic tumor microenvironment. Secondly, after being engulfed by tumor cells, SKB264 releases T030 via enzymatic cleavage. Thirdly, T030 can also penetrate the cell membrane to exert a "bystander effect" to kill surrounding tumor cells. With a high-affinity monoclonal antibody, highly active toxin molecule, good stability, and high DAR value, the overall design of SKB264 contributes to its robust anti-tumor activity which has been preliminarily demonstrated in clinical studies to date.
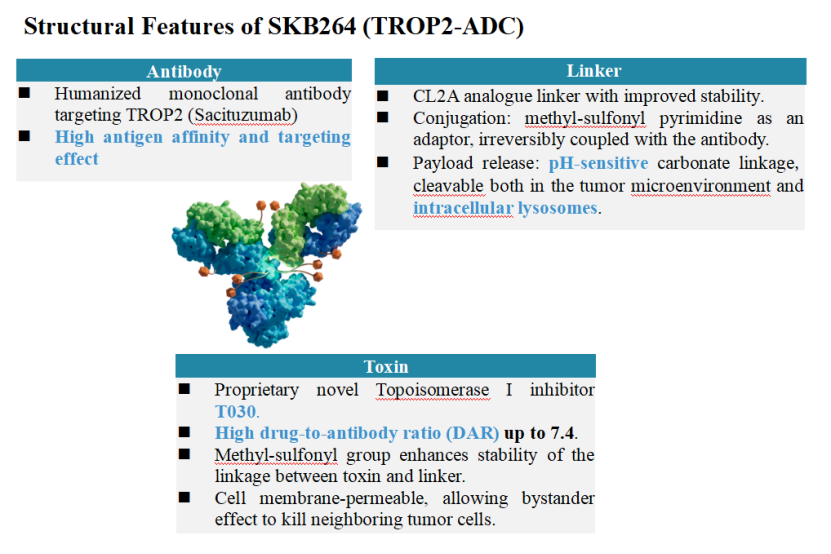
SKB264 has received 3 Breakthrough Therapy Designations (BTDs) from the Center for Drug Evaluation (CDE) of China’s National Medical Products Administration (NMPA) for the treatment of locally advanced or metastatic triple-negative breast cancer (TNBC), locally advanced or metastatic EGFR-mutated non-small cell lung cancer which has failed EGFR-TKI therapy, and locally advanced or metastatic hormone receptor-positive (HR+) and human epidermal growth factor receptor 2-negative (HER2-) breast cancer which has received at least second-line systemic therapy.
Based on preliminary clinical data, SKB264 is currently being evaluated in Phase Ⅱ and Phase Ⅲ clinical trials as either a single agent or combination therapy in multiple solid tumors.
Domestic development:
· the Phase Ⅲ pivotal clinical study of SKB264 monotherapy in patients with advanced or metastatic TNBC who have failed at least second-line therapy has reached the primary endpoint and is expected to be the first domestic TROP2-ADC to be approved for marketing.
· Clinical trial registration of SKB264 monotherapy in patients with TKI-resistant EGFR-mutated NSCLC are rapidly advancing.
· Several Phase Ⅱ clinical studies in combination with pembrolizumab (KEYTRUDA?, MSD’s anti-PD-1 therapy) or KL-A167 (Kelun’s anti-PD-L1 monoclonal antibody) are advancing steadily.
Developments abroad:
Kelun-Biotech has granted MSD the exclusive right to develop, manufacture, and commercialize SKB264 (MK-2870) in all territories outside of Greater China (includes Mainland China, Hong Kong, Macao, and Taiwan).
About Kelun-Biotech
Kelun-Biotech(6990.HK)is a holding subsidiary of Kelun Pharmaceutical (002422.SZ), which focuses on the R&D, manufacturing, commercialization and global collaboration of innovative biological drugs and small molecule drugs. The company focuses on major disease areas such as solid tumors, autoimmune, inflammatory, and metabolic diseases, and in establishing a globalized drug development and industrialization platform to address the unmet medical needs in China and the rest of world. The Company is committed to becoming a leading global enterprise in the field of innovative drugs.
At present, the Company has 33 ongoing innovative projects in major disease areas such as solid tumors, autoimmune, inflammatory, and metabolic diseases, including 14 projects in the clinical stage with several global trials being conducted simultaneously in multiple countries, including China, Europe, and the United States. The company has established one of the world’s leading proprietary ADC platforms, OptiDC, and has four ADC projects in the clinical stage (two of which are in the Phase III or NDA stage) and several projects in the preclinical stage. For more information, please visit https://kelun-biotech.com/.
Forward-Looking Statements
This press release contains certain forward-looking statements. These statements are based on the beliefs of our management as well as assumptions made by and information currently available to our management. When used in this press release, the words “aim,” “anticipate,” “believe,” “could,” “estimate,” “expect,” “going forward,” “intend,” “may,” “might,” “ought to,” “plan,” “potential,” “predict,” “project,” “seek,” “should,” “will,” “would” and the negative forms of these words and other similar expressions are intended to identify forward-looking statements. Such statements reflect the current views of our management with respect to future events, operations, liquidity and capital resources, some of which may not materialize or may change.
It is advised not to place any undue reliance on any forward-looking statements contained herein. The Company can give no assurance that these forward-looking statements will prove to have been correct. Expectations reflected in these forward-looking statements are subject to change and the Company undertakes no obligation to update or revise any forward-looking statements herein.
2023-11-27
More
Kelun-Biotech announces that KL590586 (EP0031) (small molecule RET kinase inhibitor, also named A400) program, licensed to global ex-China partner Ellipses Pharma, has been granted Orphan Drug Designation (ODD) by the US Food and Drug Administration (FDA) for the treatment of RET fusion-positive solid tumors.
Orphan drugs are drugs used for the prevention, treatment and diagnosis of rare diseases. The FDA grants ODD for investigational treatments for rare diseases, such as RET fusion-positive solid tumours, defined as affecting fewer than 200,000 people in the United States. The designation is an important milestone in the development of innovative drugs, it is expected to accelerate the progress of clinical trials in the United States. ODD qualifies the developer for certain incentives with the goal of accelerating drug development for patients, including tax credits and seven years of market exclusivity in the US upon approval by the FDA.
A400 (EP0031) is a next generation selective RET inhibitor (SRI) with broad activity against common RET fusions and mutations. Therefore, A400 (EP0031) may overcome resistance mechanisms to first generation SRIs. In preclinical studies, A400 (EP0031) demonstrated favourable inhibitory activity against key RET kinases in-vitro and in-vivo. A400 (EP0031) also demonstrated good penetration of the blood brain barrier in animal models. At present, Kelun-Biotech is conducting A400 (EP0031) pivotal clinical study in China for RET-positive non-small cell lung cancer.
In March 2021, Kelun-Biotech granted Ellipses an exclusive license for A400 (EP0031) in certain territories including the US and Europe, with Kelun-Biotech retaining certain rights in Greater China and parts of the Asia-Pacific region such as Korea, Singapore, Malaysia.
In June 2022, the FDA approved an investigational new drug application for A400 (EP0031), and a Phase 1/2 trial is ongoing in patients with malignant tumors with RET gene alteration.
About RET altered malignancies
RET fusions and mutations are present in a wide range of tumors, including non-small cell lung cancer, medullary thyroid cancer and other types of thyroid cancer, as well as colorectal cancer, so targeting RET gene changes is promising as a treatment for all types of cancer. However, for patients with RET gene alteration, the effect of traditional chemotherapy and immunotherapy is limited, especially for patients with drug resistance after first-generation SRI treatment, the acceptable treatment options are limited, and the prognosis is poor, and there are still unmet clinical needs.
About Kelun-Biotech
Kelun-Biotech(6990.HK)is a holding subsidiary of Kelun Pharmaceutical (002422.SZ), which focuses on the R&D, manufacturing, commercialization and global collaboration of innovative biological drugs and small molecule drugs. The company focuses on major disease areas such as solid tumors, autoimmune, inflammatory, and metabolic diseases, and establishing an globalized drug development and industrialization platform for the unmet medical needs in China and worldwide. The Company is committed to becoming an leading global enterprise in the field of innovation drugs.
At the present, the company has 33 ongoing innovative projects in major disease areas such as solid tumors, autoimmune, inflammatory, and metabolic diseases, including 14 projects in the clinical stage with multiple global trials conducted simultaneously in several regions including China, Europe, and the United States. The company has established one of the world’s leading in-house developed ADC platform, OptiDC, and has four ADC projects in the clinical stage (two of which are in the phase III or NDA stage, respectively) and several projects in the preclinical stage.
For more information, please visit https://kelun-biotech.com/.
About Ellipses Pharma Limited
Ellipses Pharma is a global drug development company based in London, focused on accelerating the development of cancer treatments through an innovative drug development model that combines unbiased vetting to de-risk initial asset selection with an uninterrupted funding flow to minimise the time it takes to advance lead products through clinical trials and reach patients. For more information, please visit www.ellipses.life
2023-11-13
More
First Patient Completed Dose Administration in Phase III Registrational Clinical Study of Kelun-Biotech SKB264 for the Treatment of Second-line and Above HR+/HER2- Breast Cancer
Sichuan Kelun-Biotech Biopharmaceutical Co., Ltd. (otherwise known as Kelun-Biotech, stock code: 6990.HK) completed the first patient dose administration in China for its Phase III registrational study of the innovative TROP2 ADC product, SKB264 (also known as MK-2870) recently. This study, led by Professor Xu Binghe from the Cancer Hospital of Chinese Academy of Medical Sciences, is investigating the use of SKB264 for the treatment of second-line and above hormone receptor-positive (HR+) and human epidermal growth factor receptor 2-negative (HER2-) breast cancer.
SKB264 (MK-2870), which is jointly developed with MSD (the tradename of Merck & Co., Inc., Rahway, NJ, USA), is one of the core products of Kelun-Biotech and possesses independent intellectual property rights, is the first domestically produced TROP2-ADC to secure Breakthrough Therapy Designation (BTD) from the Center for Drug Evaluation (CDE) of the National Medical Products Administration (NMPA) for the treatment of HR+/HER2- breast cancer and has subsequently entered into Phase III registrational studies. Accordingly, Kelun-Biotech has carefully designed a comprehensive development plan for this core asset in China. Currently, SKB264 monotherapy is being investigated in Phase III registrational clinical studies for HR+/HER2- breast cancer patients in the second-line setting and beyond. Additionally, SKB264 in combination or uncombination with A167 (anti-PD-L1 monoclonal antibody) is undergoing Phase II investigational studies in first-line patients. In addition, SKB264 for patients with unresectable locally advanced or metastatic triple-negative breast cancer (TNBC) who have received at least 2 prior systemic therapies, with at least 1 of those therapies targeting an advanced or metastatic stage, has been approved for in Priority Review by the CDE.
According to the latest data of the International Agency for Research on Cancer (IARC), 2.3 million new cases of breast cancer occurred worldwide in 2020, accounting for about 25% of female malignancies. Surpassing lung cancer for the first time, it is becoming the most common malignant tumor and poses a serious threat to the health of women worldwide. The annual number of new cases of female breast cancer in China (416,000) is far higher than that in other countries, ranking first in the world. Approximately 60% of breast cancer patients in China have tumors that express HR+/HER2-, and about 70%[1-2] with a higher proportion abroad. As SKB264 (MK-2870) progressively advances into the Phase III registrational clinical study for second-line and above HR+/HER2- breast cancer, and successfully administered the initial patient dosage, this provides an encouraging indication that SKB264 (MK-2870) is poised to benefit a larger population of HR+/HER2- breast cancer patients in the near future.
About SKB264 (MK-2870)
SKB264 is an innovative TROP2-directed ADC which was developed by OptiDC, a well-known international ADC R&D platform of Kelun-Biotech, using a proprietary payload-linker strategy (Kthiol design strategy) that achieves an optimized balance of ADC safety and efficacy by combining novel irreversible antibody conjugation chemistry, pH-sensitive payload release mechanisms, and site-specific moderately potent toxin molecules with a DAR of 7.4 (novel topoisomerase I inhibitors) [3].

SKB264 has received 3 Breakthrough Therapy Designations (BTDs) from the Center for Drug Evaluation (CDE) of China’s National Medical Products Administration (NMPA) for the treatment of locally advanced or metastatic triple-negative breast cancer (TNBC), locally advanced or metastatic EGFR-mutated non-small cell lung cancer which has failed EGFR-TKI therapy, and locally advanced or metastatic hormone receptor-positive (HR+) and human epidermal growth factor receptor 2-negative (HER2-) breast cancer which has received at least second-line systemic therapy.
Based on preliminary clinical data, SKB264 is currently being evaluated in Phase Ⅱ and Phase Ⅲ clinical trials as either a single agent or combination therapy in multiple solid tumors.
Domestic development:
the Phase Ⅲ registrational clinical study of SKB264 monotherapy in patients with advanced or metastatic TNBC who have failed at least second-line therapy has arrived the primary endpoint and is expected to be the first domestic TROP2-ADC to be approved for marketing.
Phase III clinical studies of SKB264 monotherapy in patients with TKI-resistant EGFR-mutated NSCLC are rapidly advancing.
Several Phase Ⅱ clinical studies in combination with pembrolizumab (KEYTRUDA?, MSD’s anti-PD-1 therapy) or KL-A167 (Kelun’s anti-PD-L1 monoclonal antibody) are conducting smoothly.
Developments abroad:
Kelun-Biotech has granted MSD the exclusive rights to develop, manufacture, and commercialize SKB264 (MK-2870) in all territories outside of Greater China (includes Mainland China, Hong Kong, Macao, and Taiwan). Currently, MSD has initiated a clinical study of MK-2870 EGFR-mutant NSCLC, and several other global multicenter registrational clinical studies are under preparation.
About Kelun-Biotech
Kelun-Biotech(6990.HK)is a subsidiary of Kelun Pharmaceutical Holdings, which focuses on the R&D, manufacture, commercialization and international cooperation of biotechnology-derived pharmaceuticals and innovative small molecule drugs. The company focuses on major disease areas such as solid tumors, autoimmune, inflammatory, and metabolic disease. Building an international drug development and industrialization platform around the unmet clinical needs of the world and China, the Company is committed to becoming an internationally leading enterprise in the field of innovation. Significant progress has been made in the field of biotechnological drugs, including ADCs, monoclonal antibodies, bispecific antibodies, and sought-after technologies for innovative small molecule drugs. OptiDC, an internationally renowned ADC research and development platform, has been successfully constructed, and 4 ADCs are in clinical trials (including 2 in the registrational Phase III or NDA filing stage), along with several projects in preclinical development. At present, the company has 33 innovative projects for the treatment of major diseases such as solid tumors, autoimmune, inflammatory, and metabolic disease, and 14 projects are advancing in clinical studies, including multiple global multicenter clinical trials which are being conducted simultaneously in several countries including China, Europe, and the United States. To learn more, please visit https://kelun-biotech.com/ .
References:
[1] 郑莹, 等. 中国癌症杂志, 2013(08):10-18.
[2] DeSantis CE, et al. CA Cancer J Clin, 2016, 66(1):31-42.
[3] Cheng Yezhe, et al. Preclinical profiles of SKB264, a novel anti-TROP2 antibody conjugated to topoisomerase inhibitor, demonstrated promising antitumor efficacy compared to IMMU-132. Frontiers in Oncology, Vol 12, 2022.
Forward-Looking Statements
This press release contains certain forward-looking statements. These statements are based on the beliefs of our management as well as assumptions made by and information currently available to our management. When used in this press release, the words “aim,” “anticipate,” “believe,” “could,” “estimate,” “expect,” “going forward,” “intend,” “may,” “might,” “ought to,” “plan,” “potential,” “predict,” “project,” “seek,” “should,” “will,” “would” and the negative forms of these words and other similar expressions are intended to identify forward-looking statements. Such statements reflect the current views of our management with respect to future events, operations, liquidity and capital resources, some of which may not materialize or may change.
It is advised not to place any undue reliance on any forward-looking statements contained herein. The Company can give no assurance that these forward-looking statements will prove to have been correct. Expectations reflected in these forward-looking statements are subject to change and the Company undertakes no obligation to update or revise any forward-looking statements herein.
2023-10-23
More
On October 20, 2023, the European Society for Medical Oncology (ESMO) Conference grandly commenced in Madrid, Spain. The ESMO Conference is the most esteemed and influential oncology conference in Europe, and this annual event garners the participation of top-tier experts and scholars from around the globe. By disseminating the latest findings in cancer clinical research, the ESMO Conference provides a high-quality platform for dialogue and learning for oncologists and other relevant stakeholders, thereby significantly influencing the practice of oncology.
During the ESMO Conference, Kelun-Biotech and MSD had a cordial meeting in Madrid on the afternoon of October 21st, local time. The leadership team and clinical team from both parties were present at ‘this meeting.
During the meeting, the management and project teams of Kelun-Biotech and MSD deliberated on the research and development progress of the multi-ongoing clinical collaboration projects and the preclinical projects that will soon enter into clinical stage. They engaged in extensive discussions regarding the potential synergy and upcoming plans for Execution of global clinical studies for the collaboration projects. Both teams are expected to work closely together to accelerate the development of projects in the clinical stage, including the rapid expansion of indications, exploration of monotherapy and combination therapy activities, and therapeutic benefit in the early-stage setting of certain cancers. This amicable meeting has significantly bolstered the foundation of collaboration between Kelun-Biotech and MSD and considerably advanced the subsequent clinical development of the ongoing collaboration projects. In addition, there is a possibility for Kelun and MSD to explore other collaboration opportunities on new target ADC assets.
On the morning of October 22nd (local time), the ESMO Conference published the results from a Phase I/II basket study in the form of a mini-oral presentation. This study investigated the innovative TROP2-ADC (SKB264, also known as MK-2870), which is under joint development by both Kelun-Biotech and MSD, for the treatment of patients with hormone receptor-positive (HR+) and human epidermal growth factor receptor 2-negative (HER2-) metastatic breast cancer (mBC). This oral presentation is based on an abstract that was published on the ESMO official website on October 16th.
About Kelun-Biotech
Kelun-Biotech is a subsidiary of Kelun Pharmaceutical Holdings, which focuses on the R&D, manufacture, commercialization and international cooperation of biotechnology-derived pharmaceuticals and innovative small molecule drugs. The company focuses on major disease areas such as solid tumors, autoimmune, inflammatory, and metabolic disease. Building an international drug development and industrialization platform around the unmet clinical needs of the world and China, the Company is committed to becoming an internationally leading enterprise in the field of innovation. Significant progress has been made in the field of biotechnological drugs, including ADCs, monoclonal antibodies, bispecific antibodies, and sought-after technologies for innovative small molecule drugs. OptiDC, an internationally renowned ADC research and development platform, has been successfully constructed, and 4 ADCs are in clinical trials (including 2 in the registrational Phase III or NDA filing stage), along with several projects in preclinical development. At present, the company has 33 innovative projects for the treatment of major diseases such as solid tumors, autoimmune, inflammatory, and metabolic disease, and 14 projects are advancing in clinical studies, including multiple global multicenter clinical trials which are being conducted simultaneously in several countries including China, Europe, and the United States. To learn more, please visit https://kelun-biotech.com/ .
2023-09-14
More
On September 14, Sichuan Kelun-Biotech Biopharmaceutical Co., Ltd. reached an exclusive license agreement with the Affiliated Hospital of Southwest Medical University on the bone-targeting radiolabeled drug TBM-001. Kelun-Biotech will obtain the global exclusive rights to the TBM-001 project, including worldwide research, development, production, and commercial promotion, and global sublicensing rights. Kelun-Biotech will pay the Affiliated Hospital of Southwest Medical University a total of 38.5 million yuan for the initial contract payment and development milestones, as well as royalties from foreign re-licensing and net sales after product launch.
The signing ceremony was held at Nanyuan Hotel in Luzhou City, Sichuan Province. The Deputy Mayor of Luzhou City Suping Luo, the Vice President of Southwest Medical University Minhai Nie, the Party Secretary of the Affiliated Hospital of Southwest Medical University Yong Xu, the Dean Yong Jiang, the Chairman of Kelun-Biotech Gexin Liu, and the General Manager of Kelun-Biotech Junyou Ge, etc. attended the signing ceremony.
The radionuclide-drug conjugate (RDC) drug is an emerging tumor precision diagnostic and therapeutic drug developed based on radionuclide-targeting ligand molecular coupling technology. RDC uses tumor target-specific molecules as carriers to guide radionuclides to accurately target tumors and achieve brachytherapy, which has unique advantages in early diagnosis of tumors and therapeutic evaluation.TBM-001 is an innovative RDC drug independently developed by Professor CY's team from the Department of Nuclear Medicine of the Affiliated Hospital of Southwest Medical University, and intended to be used for early diagnosis of bone tumor metastasis and precision targeted therapy. According to data from clinical researches initiated by the investigators, TBM-001 has shown effectiveness and safety superior to existing radioactive drug for bone metastasis in imaging diagnosis and treatment of late-stage prostate cancer, breast cancer, lung cancer and other bone metastases of malignant tumor, and significantly improved the quality of life of patients.
Regarding this cooperation, Gexin Liu, the chairman of Kelun-Biotech, stated that radioactive drug is an industry that require high investment with a high barrier of entry. The strong alliance between a regional leading university and high-level technology company is an important measure to break through key technologies and resource links in the industry, and an effective way to promote independent innovation and achievement transformation in the field of nuclear medicine in our country. Both parties are leaders in their respective fields. We all boasts distinct advantages in nuclear medicine discovery, clinical transformation, and conjugated drugs represented by ADC, and we all have industry-leading innovative achievements. This signing marks a new stage in the strategic cooperation between the two parties, highlighting our pragmatic development philosophy and our will and belief in working together for a win-win outcome and benefiting patients in the field of targeting radionuclide-drug conjugate.
Regarding this cooperation, Junyou Ge, the general manager of Kelun-Biotech, stated that RDC has become a new hot topic for anti-tumor drug research and development following Antibody-Drug Conjugates (ADC) at present. TBM-001 is a bone-targeting RDC with differentiated advantages. The innovative technology of Affiliated Hospital of Southwest Medical University and its philosophy are very consistent with Kelun-Biotech's strategy to strengthen the innovative advantages in the field of targeted drug conjugates, so we are pleased to cooperate with the Affiliated Hospital of Southwest Medical University. The Department of Nuclear Medicine has mature platform resources in the discovery of radioactive drugs and the clinical transformation of diagnosis and treatment. Combined with Kelun-Biotech's technical advantages in the ADC field and a complete new drug research and development system, both parties shall be benefited to jointly enter the RDC frontier field with high resource and technical barriers.
Minhai Nie, the Vice President of Southwest Medical University, stated that the Department of Nuclear Medicine has deep technical experience in clinical diagnosis, treatment, research and development of radiopharmaceuticals and clinical transformation. It possesses a Class IV "Radiopharmaceuticals Use Permit" and obtained National Drug Clinical Trial Accreditation. We look forward to our cooperation based on complementary advantages and efficient collaboration to realize the commercial value of TBM-001 as soon as possible, allowing patients with bone metastases from tumors to use more effective drugs at an earlier date.
Suping Luo, the Deputy Mayor of Luzhou City, congratulates both parties on reaching a cooperation on the TBM-001 project. She mentioned that the municipal party committee and the municipal government will fully support our cooperation, and they will provide corresponding services to support the strong alliance between Kelun-Biotech and the Affiliated Hospital of Southwest Medical University to help them grow and develop. She hopes that both parties will leverage their own advantages to achieve a perfect combination of conjugate and nuclear medicine, provide new treatment techniques to ensure the health of the people, and promote the high-quality development of the bio-nuclear medicine industry in Luzhou, Sichuan, and even the whole country.
In December 2022, the Affiliated Hospital of Southwest Medical University reached an cooperation with the "National Engineering Research Center for Bio-targeted Drugs" of Kelun-Biotech. The cooperation have started in the field of radioactive drugs. In addition to TBM-001, the Company and the Affiliated Hospital of Southwest Medical University have also initiated the co-development of other RDC drugs targeting unmet clinical needs. Kelun-Biotech will work together with partners in the industry to integrate superior resources, fully utilize the technical advantages of the OptiDC platform, and accelerate the pace in tapping into the new filed of RDC.
-----------
About the Affiliated Hospital of Southwest Medical University
The affiliated hospital of Southwest Medical University was established in August 1950, located in Luzhou City, Sichuan Province. It is a tertiary general hospital directly under the Health Commission of Sichuan Provincial. It serves nearly 60 million people in the combined region of Sichuan, Chongqing, Yunnan, and Guizhou provinces. The hospital currently has two campuses, Zhongshan and Kangjian Center, and is a comprehensive clinical teaching hospital integrating medical treatment, teaching, and scientific research.
About the Nuclear Medicine Department of the Affiliated Hospital of Southwest Medical University
The Department of Nuclear Medicine at the Affiliated Hospital of Southwest Medical University is a top-tier nuclear medicine department in China. According to the big data analysis of SCI papers of nuclear medicine published worldwide in 2022 released by the authoritative organization Healsan Consulting LLC, the Affiliated Hospital of Southwest Medical University ranks third in the world and first in China in terms of the impact of its SCI papers of nuclear medicine.
About Sichuan Kelun-Biotech Biopharmaceutical Co., Ltd.
Sichuan Kelun-Biotech Biopharmaceutical Co., Ltd. (6990.HK) (hereinafter referred to as "Kelun-Biotech") is a holding subsidiary of Kelun Pharmaceutical. It is committed to the R&D, manufacturing, commercialization, and international cooperation of biotechnological drugs and innovative small molecule drugs. Kelun-Biotech has been committed to addressing medical needs in China and globally. With a key focus on major disease areas such as cancer, autoimmunity, inflammation, and metabolism, it aims to build an international drug R&D and industrialization platform, striving to become a leading innovative enterprise. Kelun-Biotech has made significant progress in the field of biotechnological drugs, including ADCs, monoclonal antibodies, bispecific antibodies, and innovative small molecule drugs with new targets. Kelun-Biotech has successfully established the internationally renowned ADC R&D platform OptiDC, with 4 ADCs investigated in clinical studies (2 of which are in the registration Phase III or NDA stage, respectively), and several ADCs in preclinical studies. Kelun-Biotech currently has 33 innovative projects for the treatment of major diseases such as cancer, autoimmunity, inflammation, and metabolism, including 14 in clinical stage. Several clinical trials are multi-center studies conducted simultaneously in multiple countries including China, Europe and America.
2022年09月06日
我们决定向甘孜泸定、雅安石棉捐赠300万元现金、300万元物资,目前已成功对接甘孜州红十字会、雅安市红十字会,今天下午已经完成打款,物资根据当地所需正在紧急集结。对于灾区需要的其他支持,我们也当全力以赴。”
9月6日下午,四川科伦药业股份有限公司相关负责人告诉记者,针对四川泸定6.8级地震中受灾严重的泸定县和石棉县,他们紧急启动灾害应急处理方案,并进行现金和物资捐赠。