我们决定向甘孜泸定、雅安石棉捐赠300万元现金、300万元物资,目前已成功对接甘孜州红十字会、雅安市红十字会,今天下午已经完成打款,物资根据当地所需正在紧急集结。对于灾区需要的其他支持,我们也当全力以赴。”
9月6日下午,四川科伦药业股份有限公司相关负责人告诉记者,针对四川泸定6.8级地震中受灾严重的泸定县和石棉县,他们紧急启动灾害应急处理方案,并进行现金和物资捐赠。
2026-01-07
The 44th J.P. Morgan Healthcare Conference (JPMHC) will be held in San Francisco, California, USA, from January 12 to 15, 2026. Sichuan Kelun-Biotech Biopharmaceutical Co., Ltd. (“Kelun-Biotech” or the “Company”, 6990.HK) has been invited to attend the conference and Dr. Michael Ge, President and CEO of Kelun-Biotech, will share the company’s latest business progresses as well as its innovation capabilities and strategies.
Presentation Time: Thursday, January 15, 2026 at 9:30 AM Pacific Standard Time
Presentation Venue: Westin St. Francis, San Francisco, USA
J.P. Morgan Annual Healthcare Conference is honored as a “benchmark for development and investment in the global healthcare area”. During the conference, Kelun-Biotech will join massive seasoned experts and industry leaders to discuss cutting-edge trends in biopharmaceutical innovation. Based on the ongoing transformation moment of the global pharmaceutical industry, the company will actively pursue mutually beneficial partnership opportunities on the international stage.
You can visit “Investor Relations - Investor Calendar” page of Kelun-Biotech’s official website for participation of live webcast of presentation session, and watch replay within 30 days after the conference.
About Kelun-Biotech
Kelun-Biotech (6990.HK) is a holding subsidiary of Kelun Pharmaceutical (002422.SZ), which focuses on the R&D, manufacturing, commercialization and global collaboration of innovative biological drugs and small molecule drugs. Kelun-Biotech focuses on major disease areas such as solid tumors, autoimmune, and metabolic diseases, and in establishing a globalized drug development and industrialization platform to address the unmet medical needs in China and the rest of world. Kelun-Biotech is committed to becoming a leading global enterprise in the field of innovative drugs. At present, Kelun-Biotech has more than 30 ongoing key innovative drug projects, of which 4 projects have been approved for marketing, 1 project is in the NDA stage and more than 10 projects are in the clinical stage. Kelun-Biotech has established one of the world's leading proprietary ADC and novel DC platforms, OptiDC™, and has 2 ADC projects approved for marketing, and multiple ADC and novel DC assets in clinical or preclinical research stage. For more information, please visit https://en.kelun-biotech.com/.
2026-01-05
CHENGDU, China, Jan. 5, 2026 /PRNewswire/ -- Sichuan Kelun-Biotech Biopharmaceutical Co., Ltd. ("Kelun-Biotech" or the "Company", 6990.HK) announced that its Investigational New Drug (IND) application for SKB105 (also known as CR-003), an internally developed integrin beta-6 (ITGB6)-targeted antibody-drug conjugate (ADC), has been approved by the Center for Drug Evaluation (CDE) of the National Medical Products Administration (NMPA) of China for the treatment of advanced solid tumors.
In December 2025, Kelun-Biotech and Crescent Biopharma, Inc. ("Crescent") entered into a strategic collaboration for SKB105/CR-003 and SKB118 (a PD-1 x VEGF bispecific antibody, also known as CR-001). Under the collaboration, Kelun-Biotech granted Crescent exclusive rights to research, develop, manufacture and commercialize SKB105/CR-003 in the United States, Europe and all other markets outside of Greater China. In addition, Crescent granted Kelun-Biotech exclusive rights to research, develop, manufacture and commercialize SKB118/CR-001 in Greater China. The IND application for SKB118/CR-001 has been cleared by the U.S. Food and Drug Administration (FDA) with a global Phase I/II clinical trial for the treatment of advanced solid tumors is set to commence shortly. Kelun-Biotech plans to submit an IND application for SKB118/CR-001 to the Center for Drug Evaluation of the National Medical Products Administration of China in the near future.
About SKB105 (also known as CR-003)
SKB105 is a differentiated ADC targeting ITGB6 with a topoisomerase I inhibitor payload. ITGB6 is overexpressed in many solid tumors, but shows minimal to no expression in most normal tissues, thereby potentially reducing the risk of systemic toxicity and off-target effects. SKB105 incorporates proprietary Kthiol® irreversible conjugation technology, linking an anti-ITGB6 fully human Immunoglobulin G1 (IgG1) monoclonal antibody (mAb) to a clinically validated cleavable linker. This design aims to enhance stability and tumor-specific payload delivery while reducing adverse effects. In preclinical models, SKB105 demonstrated a favorable efficacy, safety, and pharmacokinetic (PK) profile.
About SKB118(also known as CR-001)
SKB118/CR-001 is a tetravalent bispecific antibody being developed for the treatment of solid tumors that combines two complementary, validated mechanisms in oncology via a blockade of PD-1 and VEGF. PD-1 checkpoint inhibition is aimed at restoring T cells' ability to recognize and destroy tumor cells, and blocking VEGF is intended for reducing blood supply to tumor cells and inhibiting tumor growth. In preclinical studies, SKB118/CR-001 demonstrated cooperative pharmacology with increased binding to PD-1 and signal blockade in the presence of VEGF as well as robust anti-tumor activity. SKB118/CR-001's anti-VEGF activity may also normalize the vasculature at the tumor site, which has the potential to improve the localization and effectiveness of combination therapies, particularly in conjunction with ADCs.
About Kelun-Biotech
Kelun-Biotech (6990.HK) is a holding subsidiary of Kelun Pharmaceutical (002422.SZ), which focuses on the R&D, manufacturing, commercialization and global collaboration of innovative biological drugs and small molecule drugs. Kelun-Biotech focuses on major disease areas such as solid tumors, autoimmune, inflammatory, and metabolic diseases, and in establishing a globalized drug development and industrialization platform to address the unmet medical needs in China and the rest of world. Kelun-Biotech is committed to becoming a leading global enterprise in the field of innovative drugs. At present, Kelun-Biotech has more than 30 ongoing key innovative drug projects, of which 4 projects have been approved for marketing, 1 project is in the NDA stage and more than 10 projects are in the clinical stage. Kelun-Biotech has established one of the world's leading proprietary ADC and novel DC platforms, OptiDC™, and has 2 ADC projects approved for marketing, and multiple ADC and novel DC assets in clinical or preclinical research stage. For more information, please visit https://en.kelun-biotech.com/.
2026-01-05
CHENGDU, China, January 5, 2026 -- Sichuan Kelun-Biotech Biopharmaceutical Co., Ltd. (“Kelun-Biotech” or the “Company,” 6990.HK) today announced that its TROP2-directed antibody-drug conjugate (ADC) sacituzumab tirumotecan (sac-TMT, also known as SKB264/MK-2870) (佳泰莱®) in combination with MSD’s anti-PD-1 monoclonal antibody pembrolizumab (KEYTRUDA®[1]) was granted Breakthrough Therapy Designation (BTD) by the Center for Drug Evaluation (CDE) of the National Medical Products Administration (NMPA) of China for the first-line treatment of patients with locally advanced or metastatic non-small cell lung cancer (NSCLC) who have PD-L1 tumor proportion score (TPS)≥1% and are EGFR-negative and ALK-negative.
BTD is granted for treatment regimens that provide effective treatment or prevention for conditions with no currently available therapy, or that demonstrate significant clinical advantages over currently available treatments. For drugs included in the breakthrough therapy process, if relevant conditions are met, applications for conditional approval and priority review and approval can be submitted when applying for marketing authorization.
Previously, the company announced that results from the Phase III clinical trial of OptiTROP-Lung05, evaluating sac-TMT in combination with pembrolizumab as first-line treatment for PD-L1-positive NSCLC, demonstrated a statistically significant and clinically meaningful improvement in its primary endpoint of progression-free survival (PFS). A positive trend was also observed in overall survival (OS). OptiTROP-Lung05 is the first Phase III study of an immunotherapy and ADC combination to meet its primary endpoint in the first-line treatment of NSCLC. Granting of BTD for the first-line treatment of PD-L1-positive NSCLC indication offers pathways to expedite the review and potential approval process of sac-TMT for this indication.
To date, sac-TMT has received five BTDs for:
locally advanced or metastatic triple-negative breast cancer (TNBC) in July 2022;
locally advanced or metastatic EGFR-mutant NSCLC after progression on EGFR-TKI therapy in January 2023;
locally advanced or metastatic hormone-receptor positive (HR+) and human epidermal growth factor receptor 2-negative (HER2-) breast cancer (BC) in patients who have previously received at least two lines of systemic chemotherapy in June 2023;
first-line treatment of unresectable locally advanced, recurrent or metastatic PD-L1 negative TNBC in March 2024;
In combination with anti-PD-L1 monoclonal antibody tagitanlimab for the first-line treatment of locally advanced or metastatic non-squamous NSCLC without actionable genomic alterations in June 2025.
About sac-TMT
Sac-TMT, a core product of the Company, is a novel human TROP2 ADC in which the Company has proprietary intellectual property rights, targeting advanced solid tumors such as NSCLC, BC, gastric cancer (GC), gynecological tumors, among others. Sac-TMT is developed with a novel linker to conjugate the payload, a belotecan-derivative topoisomerase I inhibitor with a drug-to-antibody-ratio (DAR) of 7.4. Sac-TMT specifically recognizes TROP2 on the surface of tumor cells by recombinant anti-TROP2 humanized monoclonal antibodies, which is then endocytosed by tumor cells and releases the payload KL610023 intracellularly. KL610023, as a topoisomerase I inhibitor, induces DNA damage to tumor cells, which in turn leads to cell-cycle arrest and apoptosis. In addition, it also releases KL610023 in the tumor microenvironment. Given that KL610023 is membrane permeable, it can enable a bystander effect, or in other words kill adjacent tumor cells.
In May 2022, the Company licensed the exclusive rights to MSD (the tradename of Merck & Co., Inc, Rahway, NJ, USA) to develop, use, manufacture and commercialize sac-TMT in all territories outside of Greater China (which includes Mainland China, Hong Kong, Macao and Taiwan).
To date, three indications for sac-TMT have been approved and marketed in China for: EGFR mutant-positive locally advanced or metastatic non-squamous NSCLC following progression on EGFR-TKI therapy and platinum-based chemotherapy; Unresectable locally advanced or metastatic TNBC who have received at least two prior systemic therapies (at least one of them for advanced or metastatic setting); EGFR mutant-positive locally advanced or metastatic non-squamous NSCLC who progressed after treatment with EGFR-TKI therapy. The first two indications listed above have been included in China's National Reimbursement Drug List (NRDL). This inclusion is expected to bring clinical benefits to a greater number of cancer patients.
Sac-TMT is the world's first TROP2 ADC drug approved for marketing in lung cancer. In addition, the sNDA for sac-TMT for second-line and above treatment of HR+/HER2- BC was accepted by the Center for Drug Evaluation of the National Medical Products Administration, and was included in the priority review and approval process.
As of today, Kelun-Biotech has initiated 9 registrational clinical studies in China. MSD has initiated 15 ongoing Phase 3 global clinical studies of sac-TMT as a monotherapy or with pembrolizumab or other anti-cancer agents for several types of cancer. These studies are sponsored and led by MSD.
About Kelun-Biotech
Kelun-Biotech (6990.HK) is a holding subsidiary of Kelun Pharmaceutical (002422.SZ), which focuses on the R&D, manufacturing, commercialization and global collaboration of innovative biological drugs and small molecule drugs. Kelun-Biotech focuses on major disease areas such as solid tumors, autoimmune, inflammatory, and metabolic diseases, and in establishing a globalized drug development and industrialization platform to address the unmet medical needs in China and the rest of world. Kelun-Biotech is committed to becoming a leading global enterprise in the field of innovative drugs. At present, Kelun-Biotech has more than 30 ongoing key innovative drug projects, of which 4 projects have been approved for marketing, 1 project is in the NDA stage and more than 10 projects are in the clinical stage. Kelun-Biotech has established one of the world's leading proprietary ADC and novel DC platforms, OptiDC™, and has 2 ADC projects approved for marketing, and multiple ADC and novel DC assets in clinical or preclinical research stage. For more information, please visit https://en.kelun-biotech.com/.
[1] KEYTRUDA® (pembrolizumab) is a registered trademark of Merck Sharp & Dohme LLC (MSD), a subsidiary of Merck & Co., Inc., Rahway, NJ, USA.
2025-12-04
Companies to advance CR-001, a PD-1 x VEGF bispecific antibody, and SKB105, an integrin beta-6-directed antibody-drug conjugate (ADC), in global markets and China
Collaboration designed to accelerate and expand the development of synergistic combinations with CR-001 and ADCs, including SKB105
CR-001 and SKB105 on track to enter Phase 1/2 monotherapy clinical trials in Q1 2026 with combination studies to follow
CHENGDU, China and WALTHAM, Mass., Dec. 4, 2025 /PRNewswire/ -- Sichuan Kelun-Biotech Biopharmaceutical Co., Ltd. ("Kelun-Biotech", 6990.HK), which focuses on the R&D, manufacturing, commercialization and global collaboration of innovative biological drugs and small molecule drugs, and Crescent Biopharma, Inc. ("Crescent") (Nasdaq: CBIO), a biotechnology company dedicated to rapidly advancing the next wave of therapies for cancer patients, today announced that the companies have entered into a strategic partnership to develop and commercialize oncology therapeutics, including novel combinations.
The partnership involves Crescent's CR-001, a PD-1 x VEGF bispecific antibody, and Kelun-Biotech's SKB105, an integrin beta-6 (ITGB6)-directed antibody-drug conjugate (ADC) with a topoisomerase payload. Both candidates are being developed for the treatment of solid tumors and are expected to enter Phase 1/2 monotherapy clinical trials in the first quarter of 2026.
Under the terms of the collaboration, Crescent has granted Kelun-Biotech exclusive rights to research, develop, manufacture and commercialize CR-001 in Greater China (including mainland China, Hong Kong, Macau and Taiwan). In addition, Kelun-Biotech has granted Crescent exclusive rights to research, develop, manufacture and commercialize SKB105 in the United States, Europe and all other markets outside of Greater China. The partnership includes the development of these candidates as monotherapies, and also the evaluation of CR-001 in combination with SKB105.Both Crescent and Kelun-Biotech have the right to independently develop CR-001 in additional combinations, including combinations of CR-001 with proprietary ADC pipeline assets.
Dr. Michael Ge, chief Executive officer of Kelun-Biotech, said, "We are pleased to have entered into a partnership with Crescent for two innovative assets, CR-001 and SKB105. This collaboration complements and strengthens our differentiated oncology pipeline by the addition of CR-001 and also enables us to advance the development of SKB105 in the global market, bolstering its potential commercial value and our global collaboration network. Our creative global partnership combines the capabilities of both companies to explore novel monotherapies and combination strategies for tumor treatments with SKB105 and CR-001. By leveraging China's abundant clinical resources and Execution efficiency, we aim to expedite clinical development while rigorously maintaining the highest global standards. We believe this partnership creates a powerful synergy to maximize the potential of these two drug candidates for the treatment of patients in both China and the rest of the world."
"We are thrilled to be partnering with Kelun-Biotech, an established leader in the development and commercialization of ADCs who shares our commitment to bringing next generation therapeutics that can improve outcomes for people living with cancer," said Joshua Brumm, chief Executive officer of Crescent. "This collaboration expands our pipeline with the addition of SKB105, furthers our strategy of advancing multiple modalities across our portfolio, and accelerates our efforts to deliver synergistic combinations with CR-001, which has the potential to be a foundational backbone therapy. We look forward to working with Kelun-Biotech to drive innovative therapeutics with the potential to address multiple tumor types and transform cancer care."
Under the collaboration, Kelun-Biotech will receive an upfront payment of US$80 million from Crescent and is also eligible to receive additional milestones of up to US$1.25 billion, plus tiered middle single-digit to low double-digit royalties on net sales of SKB105. Kelun-Biotech is also eligible to receive additional payment from Crescent if Crescent undergoes a near-term change of control or enters into a sublicense agreement with a third party. Crescent will receive an upfront payment of US$20 million from Kelun-Biotech and is also eligible to receive additional milestones of up to US$30 million, plus tiered low to middle single digit royalties on net sales of CR-001.
About CR-001 (also known as SKB118)
CR-001 is a tetravalent bispecific antibody being developed for the treatment of solid tumors that combines two complementary, validated mechanisms in oncology via a blockade of PD-1 and VEGF. PD-1 checkpoint inhibition is aimed at restoring T cells' ability to recognize and destroy tumor cells, and blocking VEGF is intended for reducing blood supply to tumor cells and inhibiting tumor growth. In preclinical studies, CR-001 demonstrated cooperative pharmacology with increased binding to PD-1 and signal blockade in the presence of VEGF as well as robust anti-tumor activity. CR-001's anti-VEGF activity may also normalize the vasculature at the tumor site, which has the potential to improve the localization and effectiveness of combination therapies, such as the administration of CR-001 with antibody-drug conjugates (ADCs). A global Phase 1/2 trial of CR-001 in patients with solid tumors is anticipated to commence in the first quarter of 2026.
About SKB105 (also known as CR-003)
SKB105 is a differentiated ADC targeting integrin beta-6 (ITGB6) with a topoisomerase 1 inhibitor payload. ITGB6 is overexpressed in many solid tumors, but shows minimal to no expression in most normal tissues, thereby potentially reducing the risk of systemic toxicity and off-target effects. SKB105 consists of an anti-ITGB6 fully human IgG1 monoclonal antibody conjugated via a stable, clinically validated cleavable linker. The molecule incorporates proprietary Kthiol® irreversible conjugation technology, designed to enhance stability and tumor-specific payload delivery while reducing adverse effects. SKB105 demonstrated a favorable efficacy, safety, and pharmacokinetic (PK) profile in preclinical models. A Phase 1/2 clinical trial of SKB105 in patients with solid tumors is anticipated to commence in the first quarter of 2026.
About Kelun-Biotech
Kelun-Biotech (6990.HK) is a holding subsidiary of Kelun Pharmaceutical (002422.SZ), which focuses on the R&D, manufacturing, commercialization and global collaboration of innovative biological drugs and small molecule drugs. Kelun-Biotech focuses on major disease areas such as solid tumors, autoimmune, inflammatory, and metabolic diseases, and in establishing a globalized drug development and industrialization platform to address the unmet medical needs in China and the rest of world. Kelun-Biotech is committed to becoming a leading global enterprise in the field of innovative drugs. At present, Kelun-Biotech has more than 30 ongoing key innovative drug projects, of which 4 projects have been approved for marketing, 1 project is in the NDA stage and more than 10 projects are in the clinical stage. Kelun-Biotech has established one of the world's leading proprietary ADC and novel DC platforms, OptiDC™, and has 2 ADC projects approved for marketing, and multiple ADC and novel DC assets in clinical or preclinical research stage. For more information, please visit https://en.kelun-biotech.com/.
About Crescent Biopharma
Crescent Biopharma's vision is to build a world leading oncology company bringing the next wave of therapies for cancer patients. Crescent 's pipeline includes its lead program, a PD-1 x VEGF bispecific antibody, as well as novel antibody-drug conjugates (ADCs). By leveraging multiple modalities and established targets, Crescent aims to rapidly advance potentially transformative therapies either as single agents or as part of combination regimens to treat a range of solid tumors. For more information, visit www.crescentbiopharma.com and follow Crescent on LinkedIn and X.
2025-10-20
After a median follow-up of 18.9 months, the median PFS was 8.3 months in the sac-TMT group and 4.3 months in the chemotherapy group (hazard ratio (HR), 0.49; 95% confidence interval (CI), 0.39 to 0.62; two-sided P<0.0001).
OS was significantly longer with sac-TMT than with chemotherapy (HR, 0.60; 95% CI, 0.44 to 0.82; two-sided P=0.001); 18-month OS rate was 65.8% and 48.0%, respectively. In the supplementary analysis, in which data were censored at the start date of subsequent ADC, the HR was 0.56 (95% CI, 0.41 to 0.77).
Sac-TMT was associated with a higher incidence of stomatitis with most cases being mild and with grade 3 or higher cases reported in very few patients (4.8%; all were grade 3). Low incidence of ocular-surface toxic effects, no ILD or pneumonitis, and one infusion-related reaction (grade 2) was reported in the sac-TMT group.
On October 20, results from the Phase III registrational clinical study OptiTROP-Lung04—led by Professor Zhang Li's team from Sun Yat-sen University Cancer Center—were published in the New England Journal of Medicine (Impact Factor = 78.5). The study evaluated the efficacy and safety of the TROP2 antibody-drug conjugate (ADC) sacituzumab tirumotecan (sac-TMT) monotherapy versus pemetrexed plus platinum chemotherapy for the treatment of patients with epidermal growth factor receptor (EGFR)-mutant locally advanced or metastatic non-squamous non-small cell lung cancer (NSCLC) who have progressed failed after treatment with EGFR-tyrosine kinase inhibitor (TKI) therapy. The study was also selected as a Late-Breaking Abstract (LBA) at the 2025 European Society for Medical Oncology (ESMO) Congress and was presented as an oral report in the Presidential Symposium (Presentation # LBA5).
OptiTROP-Lung04 is a randomized, open-label, multicenter Phase III study designed to evaluate the efficacy and safety of sac-TMT monotherapy versus pemetrexed plus platinum in patients with EGFR-mutated locally advanced or metastatic NSCLC who had progressed on prior EGFR-TKI therapy. Eligible patients were those with histologically and/or cytologically confirmed disease. The primary endpoint was Progression-Free Survival (PFS) assessed by a Blinded Independent Review Committee (BIRC) per RECIST v1.1, and the key secondary endpoint was overall survival (OS). Results showed that compared with platinum-based doublet chemotherapy, sac-TMT monotherapy demonstrated a statistically significant and clinically meaningful improvement in both PFS and OS. Consistent PFS and OS benefits were observed across all prespecified subgroups, including prior EGFR-TKI therapy, presence of liver or brain metastases, and EGFR mutation subtype.
Based on the positive results of the OptiTROP-Lung04 study, sac-TMT has been approved by the National Medical Products Administration (NMPA) in China for the treatment of adult patients with EGFR mutation-positive locally advanced or metastatic non-squamous NSCLC who have progressed after EGFR-TKI therapy. To date, sac-TMT monotherapy remains the only approved treatment option for advanced EGFR-mutant NSCLC patients who have progressed after prior TKI therapy, as well as those who have progressed after prior TKI and platinum-based chemotherapy (either concurrently or sequentially). This achievement provides comprehensive coverage for the entire population of TKI-resistant patients.
Dr. Michael Ge, Chief Executive Officer of Kelun-Biotech, commented: “OptiTROP-Lung04 is a key study that propels sac-TMT from late-line to an earlier-line treatment-setting in lung cancer, achieving a significant improvement in overall survival. We are thrilled that the results have been published in The New England Journal of Medicine, allowing this important finding to be shared widely among medical professionals and providing a reference for future lung cancer research. Together with our partner MSD, we will continue to advance sac-TMT’s clinical development and regulatory approvals, covering broader lung cancer and other indications, with the goal of making sac-TMT a cornerstone therapy for the benefit of patients.”
Professor Shengxiang Ren from the Department of Oncology at Shanghai Pulmonary Hospital, Tongji University, stated: “The application of third-generation EGFR-TKIs has significantly improved the overall prognosis for patients with advanced EGFR-mutant NSCLC. However, drug resistance is almost inevitable. Traditional treatment after resistance involves platinum-based doublet chemotherapy, which offers limited overall benefit. In recent years, regimens of chemotherapy plus immune checkpoint inhibitors+anti-angiogenic agents, or EGFR/c-Met bsAb, or PD-1/VEGF bsAb, have been successively approved for this indication. However, all these approaches are chemotherapy-based, highlighting an urgent need to explore novel treatment regimens. The OptiTROP-Lung04 study confirms sac-TMT as the first treatment option as a monotherapy to deliver clear dual benefits in both PFS and OS following EGFR-TKI progression. The sequential application of sac-TMT prior to chemotherapy demonstrates strong competence, establishing itself as a new standard of care for patients with advanced EGFR-mutant lung cancer after TKI resistance.”
About Sac-TMT
Sac-TMT, a core product of the Company, is a novel human TROP2 ADC in which the Company has proprietary intellectual property rights, targeting advanced solid tumors such as NSCLC, breast cancer (BC), gastric cancer (GC), gynecological tumors, among others. Sac-TMT is developed with a novel linker to conjugate the payload, a belotecan-derivative topoisomerase I inhibitor with a drug-to-antibody-ratio (DAR) of 7.4. Sac-TMT specifically recognizes TROP2 on the surface of tumor cells by recombinant anti-TROP2 humanized monoclonal antibodies, which is then endocytosed by tumor cells and releases the payload KL610023 intracellularly. KL610023, as a topoisomerase I inhibitor, induces DNA damage to tumor cells, which in turn leads to cell-cycle arrest and apoptosis. In addition, it also releases KL610023 in the tumor microenvironment. Given that KL610023 is membrane permeable, it can enable a bystander effect, or in other words kill adjacent tumor cells.
In May 2022, the Company licensed the exclusive rights to MSD (the tradename of Merck & Co., Inc., Rahway, NJ, USA) to develop, use, manufacture and commercialize sac-TMT in all territories outside of Greater China (includes Mainland China, Hong Kong, Macau, and Taiwan).
To date, three indications for sac-TMT have been approved and marketed in China for the treatment of adult patients with unresectable locally advanced or metastatic triple negative breast cancer (TNBC) who have received at least two prior systemic therapies (at least one of them for advanced or metastatic setting), EGFR mutation-positive locally advanced or metastatic non-squamous NSCLC following progression on EGFR-TKI therapy and platinum-based chemotherapy and EGFR mutant-positive locally advanced or metastatic non-squamous NSCLC who progressed after treatment with EGFR-TKI therapy. Sac-TMT is the first TROP2 ADC drug approved for marketing in lung cancer globally. In addition, the new indication application for sac-TMT for the treatment of adult patients with unresectable locally advanced, metastatic hormone receptor positive (HR+) and human epidermal growth factor receptor 2-negative (HER2-) BC who have received prior endocrine therapy and other systemic treatments in the advanced or metastatic setting was accepted by the Center for Drug Evaluation of the NMPA, and was included in the priority review and approval process.
As of today, the Company has initiated 9 registrational clinical studies in China. MSD has initiated 15 ongoing Phase Ⅲ global clinical studies of sac-TMT as a monotherapy or with pembrolizumab1 or other anti-cancer agents for several types of cancer. These studies are sponsored and led by MSD.
About Kelun-Biotech
Kelun-Biotech (6990.HK) is a holding subsidiary of Kelun Pharmaceutical (002422.SZ), which focuses on the R&D, manufacturing, commercialization and global collaboration of innovative biological drugs and small molecule drugs. The company focuses on major disease areas such as solid tumors, autoimmune, inflammatory, and metabolic diseases, and in establishing a globalized drug development and industrialization platform to address the unmet medical needs in China and the rest of world. The Company is committed to becoming a leading global enterprise in the field of innovative drugs. At present, the Company has more than 30 ongoing key innovative drug projects, of which 4 projects have been approved for marketing, 1 project is in the NDA stage and more than 10 projects are in the clinical stage. The company has established one of the world's leading proprietary ADC and novel DC platforms, OptiDC™, and has 2 ADC projects approved for marketing, and multiple ADC and novel DC assets in clinical or preclinical research stage. For more information, please visit https://kelun-biotech.com/.
References:
1. Pembrolizumab (KEYTRUDA®) is a registered trademark of Merck Sharp & Dohme LLC (MSD), a subsidiary of Merck & Co., Inc., Rahway, NJ, USA.
2025-10-19
CHENGDU, China, Oct, 18 , 2025——Sichuan Kelun-Biotech Biopharmaceutical Co., Ltd. (the “Company”) announced that at the 2025 European Society for Medical Oncology (ESMO) Congress held in Berlin, Germany, Results from a Phase 3 study of the Company’s human epidermal growth factor receptor 2 (HER2)-directed ADC trastuzumab botidotin (also known as A166) trastuzumab botidotin versus trastuzumab emtansine (T-DM1) in HER2-positive unresectable or metastatic BC was presented as an oral report by Professor Xichun Hu from Fudan University Shanghai Cancer Center (Presentation # LBA24, Proffered paper session 1: Breast cancer, metastatic).
Trastuzumab botidotin is a HER2-targeted ADC composed of a cytotoxic drug (Duostatin-5, anti-microtubule agent) with site-specific conjugation to trastuzumab via a stable protease-cleavable valine-citrulline linker. The unique linker is stable in plasma and selectively cleaved by lysosomal cathepsin B that is up-regulated in cancer cells.
In this study, a total of 365 patients with HER2+ unresectable or metastatic BC who had received at least one prior anti-HER2 therapy were randomized (1:1) to receive trastuzumab botidotin or T-DM1. 53% of patients had received ≥2 lines of anti-HER2 therapy, 61% of patients had HER2 Immunohistochemistry (IHC) 3+, and 60% of patients had been treated with TKIs, particularly pyrotinib (56%). As of April 26, 2025, median follow-up was 14.9 months.
Median PFS was significantly longer in trastuzumab botidotin than in T-DM1 (11.1 months vs 4.4 months; HR: 0.39, 95% CI, 0.30-0.51, p<0.0001). PFS benefit with trastuzumab botidotin was consistently observed regardless of prior lines of anti-HER2 therapy (HR 0.36, 95% CI, 0.25-0.53, for 1 prior line; HR 0.39, 95% CI, 0.28-0.56, for ≥2 prior lines).
ORR by blinded independent central review (BICR) was 76.9% vs 53.0%.
A trend toward benefit in OS was observed in trastuzumab botidotin (HR 0.62; 95% CI, 0.38-1.03).
Grade ≥3 treatment emergent adverse events (TEAEs) occurred in 69.8% of patients in trastuzumab botidotin and 63.7% in T-DM1. The most common TEAEs associated with dose reduction were ocular AEs for trastuzumab botidotin, and were platelet count decreased for trastuzumab emtansine. Only two patients permanently discontinued trastuzumab botidotin due to TEAE. No on-treatment deaths were observed in trastuzumab botidotin, compared with 1.6% in T-DM1, all of which were considered unrelated to treatment.
As a conclusion, this second head-to-head trial comparing T-DM1 with other anti-HER2 regimens demonstrated that trastuzumab botidotin statistically improved PFS with an ORR of 76.9% vs 53.0%. PFS benefit with trastuzumab botidotin was consistently observed regardless of prior lines of anti-HER2 therapy. Ocular AEs were also manageable.
"Professor Xichun Hu, National Lead Principal Investigator from Fudan University Shanghai Cancer Center:“Trastuzumab botidotin effectively balances safety and efficacy through its unique molecular design, reducing the incidence of interstitial lung disease and hematologic toxicity. According to research data, trastuzumab botidotin demonstrated significant survival benefits in the pivotal Phase III trial, with an overall manageable safety profile, providing a new important treatment option for pretreated HER2+ BC patients. These positive results also offer robust evidence-based support for personalized treatment and updates to clinical practice guidelines.”
About Trastuzumab botidotin
Trastuzumab botidotin is a differentiated HER2 ADC to treat advanced HER2+ solid tumors. As an innovative HER2 ADC developed by the Company, it conjugates a novel, monomethyl auristatin F (MMAF) derivative (a highly cytotoxic tubulin inhibitor, Duo-5) via a stable, enzyme-cleavable linker to a HER2 monoclonal antibody with a DAR of 2. Trastuzumab botidotin specifically binds to HER2 on the surface of tumor cells and is internalized by tumor cells, releasing the toxin molecule Duo-5 inside the cell. Duo-5 induces tumor cell cycle arrest in the G2/M phase, leading to tumor cell apoptosis. After targeting HER2, trastuzumab botidotin can also inhibit the HER2 signaling pathway; it has antibody-dependent cell-mediated cytotoxicity (ADCC) activity.
Based on the results of a multi-center, randomized, open-label, controlled, Phase 3 KL166-III-06 study, trastuzumab botidotin was approved for marketing by the NMPA in for adult patients with unresectable or metastatic HER2 positive BC who have received one or more prior anti-HER2 therapy. At a pre-specified interim analysis, trastuzumab botidotin demonstrated a statistically significant and clinically meaningful improvement in the primary endpoint of PFS as assessed by the BICR compared with T-DM1[; the beneficial trend for OS of trastuzumab botidotin was also observed.
Currently, the Company has initiated an open, multi-center Phase 2 clinical study of trastuzumab botidotin in the treatment of HER2+ unresectable or metastatic BC that previously received a topoisomerase inhibitor ADC.
About Kelun-Biotech
Kelun-Biotech (6990.HK) is a holding subsidiary of Kelun Pharmaceutical (002422.SZ), which focuses on the R&D, manufacturing, commercialization and global collaboration of innovative biological drugs and small molecule drugs. The company focuses on major disease areas such as solid tumors, autoimmune, inflammatory, and metabolic diseases, and in establishing a globalized drug development and industrialization platform to address the unmet medical needs in China and the rest of world. The Company is committed to becoming a leading global enterprise in the field of innovative drugs. At present, the Company has more than 30 ongoing key innovative drug projects, of which 4 projects have been approved for marketing, 1 projects are in the NDA stage and more than 10 projects are in the clinical stage. The company has established one of the world's leading proprietary ADC and novel DC platforms, OptiDC™, and has 1 ADC project approved for marketing and multiple ADC and novel DC assets in clinical or preclinical research stage. For more information, please visit https://kelun-biotech.com/.
2025-10-18
CHENGDU, China, Oct, 19, 2025——Sichuan Kelun-Biotech Biopharmaceutical Co., Ltd. (the “Company”) announced that at the 2025 European Society for Medical Oncology (ESMO) Congress held in Berlin, Germany, results from a Phase 3 OptiTROP-Lung04 trial of the Company’s trophoblast cell-surface antigen 2 (TROP2)-directed antibody-drug conjugate (ADC) sacituzumab tirumotecan (sac-TMT) in EGFR-mutated non-small cell lung cancer (NSCLC) following progression on epidermal growth factor receptor-tyrosine kinase inhibitors (EGFR-TKIs) was presented as an oral report by Professor Li Zhang from Sun Yat-sen University Cancer Center (Presentation # LBA5, Presidential Symposium II) and were simultaneously published in the New England Journal of Medicine (Impact Factor = 78.5).
In the OptiTROP-Lung04 trial, a total of 376 patients were randomized (1:1) to receive sac-TMT monotherapy or chemotherapy.
As at the data cut-off date July 06, 2025, the median follow-up is 18.9 months. the median Progression-Free Survival (PFS) was 8.3 months in the sac-TMT group and 4.3 months in the chemotherapy group. Sac-TMT significantly improved PFS over chemotherapy with 51% lower risk of disease progression or death (hazard ratio (HR) 0.49; 95% confidence interval (CI), 0.39-0.62; P<0.0001).
At the preplanned interim analysis of overall survival (OS), the OS was not reached (NR) in the sac-TMT group and 17.4 months in the chemotherapy group. sac-TMT significantly improved OS over chemotherapy with 40% lower risk of death (hazard ratio (HR) 0.6; 95% CI: 0.44-0.82; two-sided P=0.001). In the supplemental analysis, when censoring patients at the date of initiation of subsequent ADCs, sac-TMT significantly improved OS over chemotherapy with 44% lower risk of death (HR, 0.56; 95% CI, 0.41 - 0.77).
Sac-TMT significantly improved ORR as compared to chemotherapy (60.6% vs 43.1%)
A consistent PFS and OS benefit of sac-TMT over chemotherapy was observed across all predefined subgroups, including prior EGFR-TKI therapy, presence of liver or brain metastases, and EGFR mutation subtype.
The incidence of any grade treatment-related adverse events (TRAEs) and grade ≥3 TRAEs was similar between the two groups. The most common TRAEs for both sac-TMT and chemotherapy were hematologic toxicities. No TRAEs led to discontinuation or death, and no cases of interstitial lung disease/pneumonitis were reported in the sac-TMT group. Ocular surface toxicity: occurred in 9.6% of patients in the sac-TMT group, all of which were grade 1 - 2.
As a conclusion, sac-TMT demonstrates highly statistically significant and clinically meaningful improvements in PFS and OS compared to platinum-based chemotherapy and showed a manageable safety profile, with no unexpected safety signals identified. Several global phase 3 studies of sac-TMT monotherapy (NCT06305754, NCT06074588) and combination study with osimertinib in China (NCT06670196) in EGFR-mutant NSCLC are ongoing.
Professor Zhang Li, National Lead Principal Investigator from Sun Yat-sen University Cancer Center, commented: "Compared to platinum-based doublet chemotherapy, sac-TMT not only significantly prolonged PFS but also demonstrated a statistically significant and clinically meaningful improvement in OS within this patient population. This achievement marks a major breakthrough in global lung cancer treatment—sac-TMT, as a monotherapy, demonstrated statistically significant and clinically meaningful improvements in both PFS and OS in the Phase III trial for patients with EGFR-TKI-resistant NSCLC. This study provides highly valuable, new evidence-based guidance for lung cancer management worldwide and has the potential to reshape the therapeutic landscape for EGFR-TKI-resistant NSCLC "
About sac-TMT
Sac-TMT, a core product of the Company, is a novel human TROP2 ADC in which the Company has proprietary intellectual property rights, targeting advanced solid tumors such as NSCLC, BC, GC, gynecological tumors, among others. Sac-TMT is developed with a novel linker to conjugate the payload, a belotecan-derivative topoisomerase I inhibitor with a drug-to-antibody-ratio (DAR) of 7.4. Sac-TMT specifically recognizes TROP2 on the surface of tumor cells by recombinant anti-TROP2 humanized monoclonal antibodies, which is then endocytosed by tumor cells and releases the payload KL610023 intracellularly. KL610023, as a topoisomerase I inhibitor, induces DNA damage to tumor cells, which in turn leads to cell-cycle arrest and apoptosis. In addition, it also releases KL610023 in the tumor microenvironment. Given that KL610023 is membrane permeable, it can enable a bystander effect, or in other words kill adjacent tumor cells.
In May 2022, the Company licensed the exclusive rights to MSD (the tradename of Merck & Co., Inc, Rahway, NJ, USA) to develop, use, manufacture and commercialize sac-TMT in all territories outside of Greater China (which includes Mainland China, Hong Kong, Macao and Taiwan).
To date, three indications for sac-TMT have been approved and marketed in China for the treatment of adult patients with unresectable locally advanced or metastatic triple negative breast cancer (TNBC) who have received at least two prior systemic therapies (at least one of them for advanced or metastatic setting), EGFR mutation-positive locally advanced or metastatic non-squamous NSCLC following progression on EGFR-TKI therapy and platinum-based chemotherapy and EGFR mutant-positive locally advanced or metastatic non-squamous NSCLC who progressed after treatment with EGFR-TKI therapy. Sac-TMT is the first TROP2 ADC drug approved for marketing in lung cancer globally. In addition, the new indication applications for sac-TMT for the treatment of adult patients with unresectable locally advanced, metastatic HR+/HER2- BC who have received prior endocrine therapy and other systemic treatments in the advanced or metastatic setting was accepted by the Center for Drug Evaluation (CDE) of the National Medical Products Administration (NMPA), and was included in the priority review and approval process.
As of today, the Company has initiated 9 registrational clinical studies in China. MSD has initiated 15 ongoing Phase 3 global clinical studies of sac-TMT as a monotherapy or with pembrolizumab or other anti-cancer agents for several types of cancer. These studies are sponsored and led by MSD.
About Kelun-Biotech
Kelun-Biotech (6990.HK) is a holding subsidiary of Kelun Pharmaceutical (002422.SZ), which focuses on the R&D, manufacturing, commercialization and global collaboration of innovative biological drugs and small molecule drugs. The company focuses on major disease areas such as solid tumors, autoimmune, inflammatory, and metabolic diseases, and in establishing a globalized drug development and industrialization platform to address the unmet medical needs in China and the rest of world. The Company is committed to becoming a leading global enterprise in the field of innovative drugs. At present, the Company has more than 30 ongoing key innovative drug projects, of which 4 projects have been approved for marketing, 1 project is in the NDA stage and more than 10 projects are in the clinical stage. The company has established one of the world's leading proprietary ADC and novel DC platforms, OptiDC™, and has 2 ADC project approved for marketing and multiple ADC and novel DC assets in clinical or preclinical research stage. For more information, please visit https://kelun-biotech.com/.
2025-10-18
CHENGDU, China, Oct, 18 , 2025——Sichuan Kelun-Biotech Biopharmaceutical Co., Ltd. (the “Company”) announced that at the 2025 European Society for Medical Oncology (ESMO) Congress held in Berlin, Germany, results from a Phase 3 OptiTROP-Breast02 study of the Company’s trophoblast cell-surface antigen 2 (TROP2)-directed antibody-drug conjugate (ADC) sacituzumab tirumotecan (sac-TMT) (佳泰莱®) in previously treated locally advanced or metastatic hormone receptor-positive (HR+) and human epidermal growth factor receptor 2 -negative (HER2-) breast cancer (BC) was presented as an oral report by Professor Man Li from the Second Affiliated Hospital of Dalian Medical University (Presentation # LBA23, Proffered paper session 1: Breast cancer, metastatic). Previously, the new indication applications for sac-TMT for this indication was accepted by the National Medical Products Administration (NMPA) and was included in the priority review and approval process.
In the OptiTROP-Breast02 study, a total of 399 patients with HR+/HER2- BC who had progression on CDK4/6 inhibitors and received at least one prior line of chemotherapy in the advanced/metastatic setting were randomized (1:1) to receive sac-TMT or investigator's choice of chemotherapy (ICC).
As at the data cut-off date January 22, 2025, the median PFS was significantly longer in sac-TMT than in ICC (8.3 vs. 4.1 months; HR, 0.35; 95% CI, 0.26-0.48; P<0.0001). Clinical benefit was seen in sac-TMT independent of HER2 expression (HR for PFS in HER2-zero: 0.39, 95% CI, 0.26-0.57; in HER2-low: 0.31, 95% CI, 0.20-0.48).
Sac-TMT showed longer DoR versus chemotherapy; and ORR was also superior with sac-TMT to ICC.
There was a trend in OS that favored sac-TMT over ICC (HR, 0.33; 95% CI, 0.18-0.61).
Grade ≥ 3 TRAEs occurred in 62.0% and 64.8% of patients in sac-TMT and ICC. TRAE led to discontinuation in 0% and 0.5% of patients; pneumonitis occurred in 1.5% and 1.0% of patients (all grade 1-2) in sac-TMT and ICC, respectively.
As a conclusion, sac-TMT demonstrated statistically significant and clinically meaningful improvement in PFS compared to chemotherapy. PFS benefit was observed across all predefined subgroups and independent of HER2 status. Sac-TMT also showed a trend toward OS benefit (HR, 0.33; 95% CI, 0.18, 0.61) and demonstrated a favorable and manageable safety profile, with no new safety signals. Currently, a Phase III clinical study of sacituzumab tirumotecan as monotherapy and in combination with pembrolizumab in patients with chemotherapy-naïve HR+/HER2- breast cancer is ongoing globally, with a planned enrollment of approximately 1,200 participants. Additionally, another Phase III registrational study in the same population is currently enrolling patients in China, with a planned enrollment of 430 participants.
Professor Man Li from the Second Affiliated Hospital of Dalian Medical University, stated:“The Phase III OptiTROP-Breast02 study confirmed that, regardless of HER2 expression status, sac-TMT demonstrated promising efficacy in previously treated HR+/HER2- BC patients. As the first Phase III clinical trial focusing exclusively on an all-Chinese population with HR+/HER2- BC, the presentation of the OptiTROP-Breast02 study data at ESMO marks a significant breakthrough in clinical research in this indications in China. The positive outcomes of this study not only provide evidence-based support for the treatment of HR+/HER2- BC but also offer a safe and reliable new treatment option for breast cancer patients.”
About sac-TMT (佳泰莱®)
Sac-TMT, a core product of the Company, is a novel human TROP2 ADC in which the Company has proprietary intellectual property rights, targeting advanced solid tumors such as NSCLC, BC, GC, gynecological tumors, among others. Sac-TMT is developed with a novel linker to conjugate the payload, a belotecan-derivative topoisomerase I inhibitor with a drug-to-antibody-ratio (DAR) of 7.4. Sac-TMT specifically recognizes TROP2 on the surface of tumor cells by recombinant anti-TROP2 humanized monoclonal antibodies, which is then endocytosed by tumor cells and releases the payload KL610023 intracellularly. KL610023, as a topoisomerase I inhibitor, induces DNA damage to tumor cells, which in turn leads to cell-cycle arrest and apoptosis. In addition, it also releases KL610023 in the tumor microenvironment. Given that KL610023 is membrane permeable, it can enable a bystander effect, or in other words kill adjacent tumor cells.
In May 2022, the Company licensed the exclusive rights to MSD (the tradename of Merck & Co., Inc, Rahway, NJ, USA) to develop, use, manufacture and commercialize sac-TMT in all territories outside of Greater China (which includes Mainland China, Hong Kong, Macao and Taiwan).
To date, three indications for sac-TMT have been approved and marketed in China for the treatment of adult patients with unresectable locally advanced or metastatic triple negative breast cancer (TNBC) who have received at least two prior systemic therapies (at least one of them for advanced or metastatic setting), EGFR mutation-positive locally advanced or metastatic non-squamous NSCLC following progression on EGFR-TKI therapy and platinum-based chemotherapy and EGFR mutant-positive locally advanced or metastatic non-squamous NSCLC who progressed after treatment with EGFR-TKI therapy. Sac-TMT is the first TROP2 ADC drug approved for marketing in lung cancer globally. In addition, the new indication applications for sac-TMT for the treatment of adult patients with unresectable locally advanced, metastatic HR+/HER2- BC who have received prior endocrine therapy and other systemic treatments in the advanced or metastatic setting was accepted by the Center for Drug Evaluation (CDE) of the National Medical Products Administration (NMPA), and was included in the priority review and approval process.
As of today, the Company has initiated 9 registrational clinical studies in China. MSD has initiated 15 ongoing Phase 3 global clinical studies of sac-TMT as a monotherapy or with pembrolizumab or other anti-cancer agents for several types of cancer. These studies are sponsored and led by MSD.
About Kelun-Biotech
Kelun-Biotech (6990.HK) is a holding subsidiary of Kelun Pharmaceutical (002422.SZ), which focuses on the R&D, manufacturing, commercialization and global collaboration of innovative biological drugs and small molecule drugs. The company focuses on major disease areas such as solid tumors, autoimmune, inflammatory, and metabolic diseases, and in establishing a globalized drug development and industrialization platform to address the unmet medical needs in China and the rest of world. The Company is committed to becoming a leading global enterprise in the field of innovative drugs. At present, the Company has more than 30 ongoing key innovative drug projects, of which 4 projects have been approved for marketing, 1 project is in the NDA stage and more than 10 projects are in the clinical stage. The company has established one of the world's leading proprietary ADC and novel DC platforms, OptiDC™, and has 2 ADC project approved for marketing and multiple ADC and novel DC assets in clinical or preclinical research stage. For more information, please visit https://kelun-biotech.com/.
2025-08-19
Sichuan Kelun-Biotech Biopharmaceutical Co., Ltd. ("Kelun-Biotech", HKEX: 6990) announced that clinical data from a Phase II study evaluating novel TROP2 antibody-drug conjugate (ADC) sacituzumab tirumotecan (sac-TMT) in combination with PD-L1 monoclonal antibody (mAb) tagitanlimab for the first-line treatment of advanced or metastatic non-small cell lung cancer (NSCLC) have been published in Nature Medicine (Impact Factor: 58.7).
The publication highlighted initial findings from the Phase II OptiTROP-Lung01 study, evaluating the efficacy and safety results of sac-TMT in combination with tagitanlimab as a first-line treatment of advanced or metastatic NSCLC patients without actionable genomic alterations. The study was led by Prof. Zhang Li's team at the Center for Cancer Prevention and Control, Sun Yat-sen University, China. Patients in Cohort 1A received sac-TMT (5 mg/kg, Q3W) plus tagitanlimab (1200 mg, Q3W) in a three-week cycle, while patients in Cohort 1B were treated with sac-TMT (5 mg/kg, Q2W) plus tagitanlimab (900 mg, Q2W) in a four-week cycle. Patients received sac-TMT in combination with tagitanlimab in a non-randomized manner until disease progression or unacceptable toxicity. Median follow-ups for Cohort 1A and Cohort 1B were 19.3 months and 13.0 months, respectively (Data cutoff date: May 27, 2024).
The study results demonstrated promising anti-tumor activity, and manageable safety of sac-TMT in combination with tagitanlimab as a first-line treatment for advanced or metastatic NSCLC patients. A total of 40 patients in Cohort 1A and 63 patients in Cohort 1B were included in the full analysis set (FAS) for efficacy assessment. In Cohort 1A and Cohort 1B, respectively, the confirmed objective response rates (ORRs) were 40.0% (95% CI: 24.9–56.7) and 66.7% (95% CI: 53.7–78.0), and the ORRs were:
44.4% and 64.7% among patients with non-squamous carcinoma, 36.4% and 69.0% with squamous carcinoma;
41.7% and 57.1% among patients with PD-L1 tumor proportion score (TPS)<1%;
38.5% and 63.2% for TPS 1–49%;
40.0% and 78.3% for TPS ≥50%.
The median progression-free survival (mPFS) for Cohort 1A was 15.4 months (95% CI: 6.7–17.9) and not reached (95% CI: 9.6–NE) for Cohort 1B.
The most common grade ≥3 treatment-related adverse events (TRAEs) for both Cohorts 1A and 1B were decreased neutrophil count, decreased white blood cell count and anemia. No treatment-related deaths were observed.
Subgroup analyses showed consistent efficacy across PD-L1 and TROP2 expression levels, as well as in both squamous and non-squamous histological subtypes.
Dr. Michael Ge, CEO of Kelun-Biotech, commented: "The OptiTROP-Lung01 study supports the promising efficacy and safety of sacituzumab tirumotecan in combination with tagitanlimab as a first-line treatment for patients with advanced NSCLC. The results were observed across PD-L1/TROP2 expression levels and histological subtypes and support the advancement potential of sac-TMT from later-line to front-line therapy. The publication of results from several studies in top-tier international journals reflects the recognition of our innovation-driven development strategy. We will continue to work to address critical clinical challenges and unmet medical needs, striving to deliver more therapeutic options and improve quality of life for patients."
About sac-TMT
Sac-TMT, a core product of the Company, is a novel human TROP2 ADC in which the Company has proprietary intellectual property rights, targeting advanced solid tumors such as NSCLC, breast cancer (BC), gastric cancer (GC), gynecological tumors among others. Sac-TMT is developed with a novel linker to conjugate the payload, a belotecan-derivative topoisomerase I inhibitor with a drug-to-antibody-ratio (DAR) of 7.4. Sac-TMT specifically recognizes TROP2 on the surface of tumor cells by recombinant anti-TROP2 humanized monoclonal antibodies, which is then endocytosed by tumor cells and releases KL610023 intracellularly. KL610023, as a topoisomerase I inhibitor, induces DNA damage to tumor cells, which in turn leads to cell-cycle arrest and apoptosis. In addition, it also releases KL610023 in the tumor microenvironment. Given that KL610023 is membrane permeable, it can enable a bystander effect, or in other words kill adjacent tumor cells.
In May 2022, the Company licensed the exclusive rights to MSD (the tradename of Merck & Co., Inc., Rahway, NJ, USA) to develop, use, manufacture and commercialize sac-TMT in all territories outside of Greater China (includes Mainland China, Hong Kong, Macao, and Taiwan).
To date, two indications for sac-TMT have been approved and marketed in China for the treatment of adult patients with unresectable locally advanced or metastatic TNBC who have received at least two prior systemic therapies (at least one of them for advanced or metastatic setting) and EGFR mutation-positive locally advanced or metastatic non-squamous NSCLC following progression on EGFR-TKI therapy and platinum-based chemotherapy. In addition, two new indication applications for sac-TMT for the treatment of adult patients with EGFR-mutant locally advanced or metastatic NSCLC who progressed after treatment with EGFR-TKI therapy and with unresectable locally advanced, metastatic HR+/HER2- BC who have received prior endocrine therapy and other systemic treatments in the advanced or metastatic setting were accepted by the CDE, and were included in the priority review and approval process. As of today, the Company has initiated 9 registrational clinical studies in China. MSD has initiated 14 ongoing Phase III global clinical studies of sac-TMT as a monotherapy or with pembrolizumab[1] or other agents for several types of cancer. These studies are sponsored and led by MSD.
About Tagitanlimab
Tagitanlimab is the first PD-L1 mAb globally to receive authorization for the first-line treatment of NPC. Previously, the NMPA has approved the marketing in China of tagitanlimab used in combination with cisplatin and gemcitabine for the first-line treatment of patients with R/M NPC and monotherapy for the treatment of patients with recurrent or metastatic NPC who have failed after prior 2L+ chemotherapy, respectively.
[1] Pembrolizumab (KEYTRUDA®) is a registered trademark of Merck Sharp & Dohme LLC (MSD), a subsidiary of Merck & Co., Inc., Rahway, NJ, USA. |
About Kelun-Biotech
Kelun-Biotech(6990.HK)is a holding subsidiary of Kelun Pharmaceutical (002422.SZ), which focuses on the R&D, manufacturing, commercialization and global collaboration of innovative biological drugs and small molecule drugs. The company focuses on major disease areas such as solid tumors, autoimmune, inflammatory, and metabolic diseases, and in establishing a globalized drug development and industrialization platform to address the unmet medical needs in China and the rest of world. The Company is committed to becoming a leading global enterprise in the field of innovative drugs. At present, the Company has more than 30 ongoing key innovative drug projects, of which 3 projects have been approved for marketing, 1 project is in the NDA stage and more than 10 projects are in the clinical stage. The company has established one of the world's leading proprietary ADC platforms, OptiDC™, and has 1 ADC project approved for marketing, 1 ADC project in NDA stage and multiple ADC and novel DC assets in clinical or preclinical research stage. For more information, please visit https://kelun-biotech.com/.
2023-09-06
More
From September 2 to 6, 2023, the 2023 China International Fair for Trade in Services (abbreviated as "CIFTIS"), with the annual theme of "Opening up Leads to Development, Cooperation Delivers the Future", was held in Beijing. Only two companies from Sichuan Province were selected, one of which was Sichuan Kelun-Biotech Biopharmaceutical Co., Ltd. (abbreviated as " Kelun-Biotech "). Kelun-Biotech was successfully selected for the 2023 CIFTIS Ten-Year Achievement Exhibition and as "Demonstration Case for Science & Technology Innovation Service".
This year's CIFTIS has set up a special area for the China Service Trade Achievement Exhibition, presenting the achievements of China's service trade development over the past 10 years. The Sichuan Comprehensive Exhibition Hall covers an area of 100 square meters. Its main thread of display is Chengdu, the innovative demonstration city, and the seven national service export bases. Based on a concentrated display of high-quality physical objects, it showcases the innovative development achievements and characteristic highlights of Sichuan's service trade.

(Kelun-Biotech's large molecular model displayed at the ten-year achievement exhibition of CIFTIS)
Kelun-Biotech reached a cooperation with the international pharmaceutical giant Merck, with upfront and milestone payments totaling up to 11.8 billion US dollars, thereby setting a new record for the amount of foreign authorization transactions for innovative drugs in China. Therefore, it was successfully included in the ten-year achievement exhibition for its historic breakthrough from a basically "importer of services" to "exporter of services".
Kelun-Biotech's "OptiDC" innovative Antibody Drug Conjugate (ADC) platform is one of the only two service demonstration cases selected in Sichuan. Kelun-Biotech's "OptiDC" innovative Antibody Drug Conjugate (ADC) platform has developed a complete ADC core component library, which can design the best combination and make effective optimization according to the biological characteristics of the target, thereby proposing the optimal ADC to meet the medical needs in various indications. With its strong R&D strength and the synergistic effect of multi-platform technology, Kelun-Biotech has become a technology exporter among the world's top biopharmaceutical companies, thus opening a new paradigm of service trade.
2023-08-28
More
Kelun-Biotech Announces Interim Results for 2023:
Global Strategic Partnership and Rich Pipeline Boost Significant Revenue Growth in the First Half of the Year
Chengdu, China, August 28, 2023 – Today, Sichuan Kelun-Biotech Biopharmaceutical Co., Ltd. (“Kelun-Biotech”, HKEX: 6990.HK), an innovative biopharmaceutical company focusing on the R&D, manufacturing, commercialization, and international cooperation of biotechnology drugs and innovative small molecule drugs, announced its interim results for the six months ended June 30, 2023.
The announcement shows that Kelun-Biotech’s revenue for the first half of 2023 amounts to approximately RMB 1,046 million, an increase of 203.3% compared to the same period last year. The increase is mainly attributable to the receipt of the non-refundable upfront payment of USD175.0 million from Merck Sharp & Dohme LLC (together with its affiliates, “MSD”) in March 2023 pursuant to the license and collaboration agreement we entered into with MSD to develop up to seven preclinical ADC (antibody drug conjugate) assets for the treatment of cancer. Thanks to the revenue from license and collaboration agreements, and provision of research and development service, Loss for the period are RMB 31.13 million ,the loss for the period has significantly narrowed by 88.5% year-on-year.
R&D and commercialization work together to contribute to the long-term value accumulation
Kelun-Biotech’s core product, SKB264, was granted a Breakthrough Therapy Designation (BTD) in January, 2023 by the National Medical Products Administration (NMPA) for EGFR-TKI failed EGFR-mutant locally advanced or metastatic NSCLC and another BTD in June for locally advanced or metastatic hormone receptor positive (HR)+/human epidermal growth factor receptor 2 negative (HER2-) breast cancer (BC) which has previously received at least 2L systemic chemotherapy. In addition, the clinical development of SKB264 made significant progress in the first half of 2023: In June, the company released the data of phase II study of SKB264 in patients with locally advanced or metastatic NSCLC at the 2023 ASCO Annual Meeting, demonstrating promising efficacy and controlled safety of SKB264; In July, achieved first-patient-in for a pivotal phase III trial of SKB264 for EGFR-mutant locally advanced or metastatic non-squamous NSCLC (TKI failure) in China; In August, the phase III clinical trial of SKB264 in patients with unresectable locally advanced, recurrent or metastatic triple negative breast cancer (TNBC) who have failed second-line or above prior standard of care met the primary endpoint.
In May 2023, Kelun-Biotech submitted an NDA to the NMPA for another core product, A166 (HER2 ADC), targeting 3L+ advanced HER2+ BC. The company is also conducting a confirmatory phase III trial in China for 2L+ advanced HER2+ BC and multiple phase Ib clinical trials in China to explore the therapeutic potential of A166 for other advanced HER2+ solid tumors, including gastric cancer and colorectal cancer.
In addition, Kelun-Biotech is making significant progress with other key products. SKB315 (Claudin 18.2 ADC) is undergoing the phase Ia clinical trial in China for patients with advanced solid tumors; The phase Ia clinical trials of SKB410 for advanced solid tumors, which also received IND approval from the NMPA, successfully enrolled the first patient in July 2023; We have completed patient enrollment of the phase III trial of A167 in combination with chemotherapy as a 1L treatment for RM-NPC; We have completed patient enrollment of the phase III trial of A167 in combination with chemotherapy as a 1L treatment for RM-NPC; We have completed patient enrolment of A140 (Cetuximab biosimilar) in November 2022 with an anticipated NDA filing with NMPA for RAS wild-type mCRC in the second half of 2023. In July, the company commenced pivotal trial of A400 (RET inhibitor) for advanced RET+ NSCLC, a trial of A400 for advanced RET+ medullary thyroid carcinoma received IND approval from the NMPA.
Considering the research and development progress of its drug pipelines, Kelun-Biotech is setting up a fully-fledged commercialization team to prepare and implement the marketing and commercialization of its strategic products. Currently, Kelun-Biotech has established a department structure within the company, consisting of various departments such as Marketing, Access and Commerce, Medical Affairs, Sales, and Strategic Planning and Commercial Excellence.
Stable global strategic partnership, Kelun-Biotech has achieved a significant revenue growth
In January 2023, MSD subscribed for the Shares in Kelun-Biotech at a consideration of
approximately USD 1 million as part of the Series B Financing. In March, the receipt of the non-refundable upfront payment of USD 175.0 million from Merck Sharp & Dohme LLC (together with its affiliates, “MSD”) in March 2023 pursuant to the license and collaboration agreement we entered into with MSD to develop up to seven preclinical ADC (antibody drug conjugate) assets for the treatment of cancer. In the first half year of 2023, we had various exchanges and visits with MSD management, and we made concerted efforts to promote the progress of the project and continued to deepen the results of strategic cooperation. Currently, clinical-stage products such as SKB264, SKB315, A410 as well as other preclinical-stage products developed in collaboration with MSD are being advanced and developed in an orderly manner as planned.
Kelun-Biotech entered into a collaboration and license agreement with Ellipses, under which it granted Ellipses an exclusive, royalty-bearing, sublicensable license to develop, manufacture and commercialize A400 in all countries excluding Greater China, North Korea, South Korea, Singapore, Malaysia and Thailand. Kelun-Biotech has received certain milestone payments from Ellipses during the Reporting Period. A clinical trial application of A400 was approved by the Spanish Agency of Medicines and Medical Devices (AEMPS) in February 2023. As of July 26, 2023, seven and four clinical research centers in the United States and Europe, respectively, were in use for A400.
Strong capital operation promotes innovation strategy
In February, 2023, Kelun-Biotech completed Series B Financing and raised over USD 200 million.
In July, 2023, Kelun-Biotech was successfully listed on the Main Board of the Stock Exchange. The net proceeds arising from the Listing and the full exercise of the Over-Allotment Option amounted to approximately HK$1454.9 million.
Dr. Ge Junyou, Executive Director and General Manager of Kelun-Biotech, commented: “The significant revenue growth in the first half of 2023 is a strong testament to Kelun-Biotech’s continuous efforts to advance its clinical value-oriented and differentiated oncology and non-oncology pipeline, enhance its drug development capabilities, continue to seek strategic partners, and optimize its operation system, among other correct strategic tactics. Looking ahead to the second half of the year, Kelun-Biotech will further advance the research and development progress of various pipelines, refine the commercialization strategies for each late-stage drug candidate, and continue to pursue a flexible strategy to capture the commercial value in major international markets, through forging synergistic license and collaboration opportunities worldwide.”
About Sichuan Kelun-Biotech Biopharmaceutical Co., Ltd.
Sichuan Kelun-Biotech Biopharmaceutical Co., Ltd. (“Kelun-Biotech”) is a holding subsidiary of Sichuan Kelun Pharmaceutical Co., Ltd, which focuses on the R&D, manufacturing, commercialization, and international cooperation of biotechnology drugs and innovative small molecule drugs. The company focuses on serious diseases such as solid tumors, autoimmune, inflammatory, and metabolic disease. Building an international drug R&D and industrialization platform to address the unmet clinical needs of the world and China, the Company is committed to becoming an international leading enterprise in the field of innovation. Significant progress has been made in the field of biotechnological drugs, including ADCs, monoclonal antibodies, bispecific antibodies, and sought-after technologies for innovative small molecule drugs. OptiDC, an internationally renowned ADC research and development platform, has been successfully constructed by Kelun-Biotech, and 4 ADCs are in clinical trials (including two in registrational Phase III or NDA filing stage), along with multiple projects in preclinical development. At present, the company has 33 innovative projects for the treatment of serious diseases such as solid tumors, autoimmune, inflammatory, and metabolic disease, and 14 programs are advancing in clinical studies, including multiple global multicenter clinical trials which are being conducted simultaneously in several countries and regions including China, Europe, and the United States. To learn more, please visit https://kelun-biotech.com/.
Forward-Looking Statements
This press release contains certain forward-looking statements. These statements are based on the beliefs of our management as well as assumptions made by and information currently available to our management. When used in this press release, the words “aim,” “anticipate,” “believe,” “could,” “estimate,” “expect,” “going forward,” “intend,” “may,” “might,” “ought to,” “plan,” “potential,” “predict,” “project,” “seek,” “should,” “will,” “would” and the negative forms of these words and other similar expressions are intended to identify forward-looking statements. Such statements reflect the current views of our management with respect to future events, operations, liquidity and capital resources, some of which may not materialize or may change.
It is advised not to place any undue reliance on any forward-looking statements contained herein. The Company can give no assurance that these forward-looking statements will prove to have been correct. Expectations reflected in these forward-looking statements are subject to change and the Company undertakes no obligation to update or revise any forward-looking statements herein.
2023-08-14
Chengdu, China, August 14, 2023 - Sichuan Kelun-Biotech Biopharmaceutical Co. Ltd. ("Kelun-Biotech", HKEX: 6990.HK) announced today that its innovative TROP2-AD...
More
Chengdu, China, August 14, 2023 - Sichuan Kelun-Biotech Biopharmaceutical Co. Ltd. ("Kelun-Biotech", HKEX: 6990.HK) announced today that its innovative TROP2-ADC (SKB264, also known as MK-2870) met the primary endpoint in the randomized, controlled, open-label, multi-center Phase III clinical trial of SKB264 for injection versus investigator selected regimens in patients with unresectable locally advanced, recurrent or metastatic triple-negative breast cancer (TNBC) who have failed second-line or above prior standard of care.
Kelun-Biotech recently held an Independent Data Monitoring Committee (IDMC) meeting to review the interim analysis data of the Phase III clinical trial of SKB264. The IDMC resolution indicated that the trial met the primary endpoint of progression-free survival (PFS) as assessed by the Independent Review Committee (IRC), and at a pre-specified interim analysis, SKB264 demonstrated a statistically significant improvement in PFS, compared with the control group receiving standardized chemotherapy. Based on the results from the interim analysis, Kelun-Biotech plans to communicate with the Center for Drug Evaluation (CDE) of National Medical Products Administration (NMPA) of China regarding the submission of a new drug application (NDA) of SKB264.
Dr. Ge Junyou, Executive Director and General Manager of Kelun-Biotech, commented, "TNBC is one of the most aggressive and prevalent subtypes of breast cancer (BC) with huge unmet medical needs. Patients with TNBC who have received second-line or later treatment have an overall survival of 5.2 to 8.4 months [1]. SKB264 has been granted breakthrough therapy designation (BTD) for the treatment of locally advanced or metastatic TNBC. For drugs included in the list of BTD, the CDE will prioritize resource allocation for communication and guidance to facilitate drug development. This will help accelerate the development and commercialization of SKB264 to provide high-quality treatment options for patients with advanced TNBC."
SKB264 is one of the core products with independent intellectual property rights of Kelun-Biotech, and will be supported by an extensive development plan. This clinical trial, which met its primary endpoint, is the first registrational Phase III study of SKB264 (MK-2870) in China. In addition, Kelun-Biotech is also advancing a Phase II trial of SKB264 with or without KL-A167 (Kelun’s anti-PD-L1 monoclonal antibody) as a first-line treatment for advanced TNBC, and another randomized, open-label, multicenter Phase III clinical study of SKB264 versus investigator’s choice regimens in patients with unresectable locally advanced, recurrent or metastatic hormone receptor-positive (HR+) and human epidermal growth factor receptor 2-negative (HER2-) BC who had failed at least one line of chemotherapy is expect to be launched in the second half of 2023.
About SKB264 (MK-2870)
SKB264 is an innovative TROP2-directed ADC which was developed by OptiDC, a well-known international ADC R&D platform of Kelun-Biotech, using a proprietary payload-linker strategy (Kthiol design strategy) that achieves an optimized balance of ADC safety and efficacy by combining novel irreversible antibody conjugation chemistry, pH-sensitive payload release mechanisms, and site-specific moderately potent toxin molecules with a DAR of 7.4 (novel topoisomerase I inhibitors) [2].
SKB264 has received 3 Breakthrough Therapy Designations (BTDs) from the Center for Drug Evaluation (CDE) of China’s National Medical Products Administration (NMPA) for the treatment of locally advanced or metastatic triple-negative breast cancer (TNBC), locally advanced or metastatic EGFR-mutated non-small cell lung cancer which has failed EGFR-TKI therapy and locally advanced or metastatic hormone receptor-positive (HR+) and human epidermal growth factor receptor 2-negative (HER2-) breast cancer which has received at least second-line systemic therapy.
SKB264 is currently conducting Phase II and Phase III clinical trials of monotherapy/combination in multiple solid tumors. In China, the Phase III registrational clinical study of SKB264 monotherapy for patients with advanced or metastatic TNBC who have failed at least second-line therapy is progressing smoothly, and SKB264 is expected to become the first domestic TROP2-ADC approved for the Chinese market. Phase III clinical studies of SKB264 monotherapy in patients with TKI-resistant and EGFR-mutated non-small cell lung cancer (NSCLC) are also rapidly advancing. Several Phase II clinical studies of SKB264 in combination with pembrolizumab (KEYTRUDA?, MSD’s anti-PD-1 therapy) or KL-A167 (Kelun’s anti-PD-L1 monoclonal antibody) are ongoing. Kelun-Biotech has licensed the exclusive rights to MSD (the tradename of Merck & Co., Inc, Rahway, NJ, USA) to develop, use, manufacture and commercialize SKB264 in all territories outside of Greater China (includes Mainland China, Hong Kong, Macao, and Taiwan).
About Sichuan Kelun-Biotech Biopharmaceutical Co., Ltd.
Sichuan Kelun-Biotech Biopharmaceutical Co., Ltd. (“Kelun-Biotech”) is a holding subsidiary of Sichuan Kelun Pharmaceutical Co., Ltd, which focuses on the R&D, manufacturing, commercialization, and international cooperation of biotechnology drugs and innovative small molecule drugs. The company focuses on serious diseases such as solid tumors, autoimmune, inflammatory, and metabolic disease. Building an international drug R&D and industrialization platform to address the unmet clinical needs of the world and China, the Company is committed to becoming an international leading enterprise in the field of innovation. Significant progress has been made in the field of biotechnological drugs, including ADCs, monoclonal antibodies, bispecific antibodies, and sought-after technologies for innovative small molecule drugs. OptiDC, an internationally renowned ADC research and development platform, has been successfully constructed by Kelun-Biotech, and 4 ADCs are in clinical trials (including two in registrational Phase III or NDA filing stage), along with multiple projects in preclinical development. At present, the company has 33 innovative projects for the treatment of serious diseases such as solid tumors, autoimmune, inflammatory, and metabolic disease, and 14 programs are advancing in clinical studies, including multiple global multicenter clinical trials which are being conducted simultaneously in several countries and regions including China, Europe, and the United States. To learn more, please visit https://kelun-biotech.com/.
References:
[1] O'Shaughnessy, Joyce, et al. Assessment of sacituzumab govitecan (SG) versus treatment of physician’s choice (TPC) cohort by agent in the phase 3 ASCENT study of patients (pts) with metastatic triple-negative breast cancer (mTNBC). (2021): 1077-1077.
[2] Cheng Yezhe, et al. Preclinical profiles of SKB264, a novel anti-TROP2 antibody conjugated to topoisomerase inhibitor, demonstrated promising antitumor efficacy compared to IMMU-132. Frontiers in Oncology, Vol 12, 2022
Forward-Looking Statements
This press release contains certain forward-looking statements. These statements are based on the beliefs of our management as well as assumptions made by and information currently available to our management. When used in this press release, the words “aim,” “anticipate,” “believe,” “could,” “estimate,” “expect,” “going forward,” “intend,” “may,” “might,” “ought to,” “plan,” “potential,” “predict,” “project,” “seek,” “should,” “will,” “would” and the negative forms of these words and other similar expressions are intended to identify forward-looking statements. Such statements reflect the current views of our management with respect to future events, operations, liquidity and capital resources, some of which may not materialize or may change.
It is advised not to place any undue reliance on any forward-looking statements contained herein. The Company can give no assurance that these forward-looking statements will prove to have been correct. Expectations reflected in these forward-looking statements are subject to change and the Company undertakes no obligation to update or revise any forward-looking statements herein.
2023-08-01
Chengdu, China, August 1, 2023 - Sichuan Kelun-Biotech Biopharmaceutical Co., Ltd. ("Kelun-Biotech", HKEX: 6990.HK) announced that it will present the data from...
More
Chengdu, China, August 1, 2023 - Sichuan Kelun-Biotech Biopharmaceutical Co., Ltd. ("Kelun-Biotech", HKEX: 6990.HK) announced that it will present the data from a Phase I/II clinical trial of the innovative TROP2-ADC (SKB264, MK-2870) jointly developed by Kelun-Biotech and MSD (the tradename of Merck & Co., Inc Rahway NJ USA) at the European Society for Medical Oncology (ESMO) Congress 2023 to be held in Madrid, Spain from October 20 to 24, 2023. The data will be presented in the form of an oral presentation and will focus on the treatment of previously-treated patients with metastatic hormone receptor-positive (HR+) and human epidermal growth factor receptor 2-negative (HER2-) breast cancer. In June of this year, SKB264 was granted Breakthrough Therapy Designation (BTD) by the Center for Drug Evaluation (CDE) of China’s National Medical Products Administration (NMPA) for the treatment of locally advanced or metastatic HR+/HER2- breast cancer which has received at least second-line systemic therapy.
The abstract of this oral presentation is titled "SKB264 (MK-2870) in previously treated hormone receptor-positive (HR+)/ HER2-negative metastatic breast cancer (mBC): results from a phase I/II, single-arm, basket trial", and the presentation number is 380MO. Details of the abstract are expected to be released on the official website of the ESMO Congress on October 16, 2023, local time.
The ESMO Congress is the most prestigious and influential oncology conference in Europe. It brings together clinicians, researchers, patient advocates, journalists and healthcare industry representatives from all around the world each year. By sharing the latest cutting-edge data, it provides unparalleled networking opportunities and high quality education for oncologists and other stakeholders.
About SKB264 (MK-2870)
SKB264 is an innovative ADC targeting TROP2 which was developed by OptiDC, a well-known international ADC R&D platform of Kelun-Biotech, using a proprietary payload-linker strategy (Kthiol design strategy) that achieves an optimized balance of ADC safety and efficacy by combining novel irreversible antibody conjugation chemistry, pH-sensitive payload release mechanisms, and site-specific moderately potent toxin molecules with DAR of 7.4 (novel topoisomerase I inhibitors) [1].
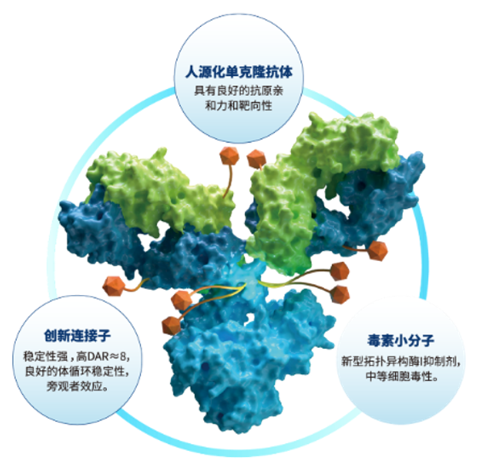
SKB264 has received 3 Breakthrough Therapy Designations (BTDs) from the Center for Drug Evaluation (CDE) of China’s National Medical Products Administration (NMPA) for the treatment of locally advanced or metastatic triple-negative breast cancer, locally advanced or metastatic EGFR-mutated non-small cell lung cancer who have failed EGFR-TKI therapy and the treatment of locally advanced or metastatic hormone receptor-positive (HR+) and human epidermal growth factor receptor 2-negative (HER2-) breast cancer which has received at least second-line systemic therapy.
SKB264 is currently conducting Phase II and Phase III clinical trials of monotherapy/combination in multiple solid tumors. In China, the Phase III registrational clinical study of SKB264 monotherapy for patients with advanced or metastatic triple-negative breast cancer (TNBC) who have failed at least second-line therapy is progressing smoothly, and it is expected to become the first domestic TROP2-ADC approved for the Chinese market. Phase III clinical studies of SKB264 monotherapy in patients with TKI-resistant and EGFR-mutated non-small cell lung cancer (NSCLC) are also rapidly advancing. Several Phase II clinical studies of SKB264 in combination with pembrolizumab (KEYTRUDA?, MSD’s anti-PD-1 therapy) or KL-A167 (Kelun’s anti-PD-L1 monoclonal antibody) are ongoing. Kelun-Biotech has licensed the exclusive rights to MSD (the tradename of Merck & Co., Inc, Rahway, NJ, USA) to research, develop, produce and commercialize SKB264 in all territories outside of Greater China (includes Mainland China, Hong Kong, Macao, and Taiwan).
About Sichuan Kelun-Biotech Biopharmaceutical Co., Ltd.
Sichuan Kelun-Biotech Biopharmaceutical Co., Ltd. (“Kelun-Biotech”) is a holding subsidiary of Sichuan Kelun Pharmaceutical Co., Ltd, which focuses on the R&D, manufacture, commercialization and international cooperation of biotechnology drugs and innovative small molecule drugs. The company focuses on serious diseases such as solid tumors, autoimmune, inflammatory, and metabolic disease. Building an international drug R&D and industrialization platform to address the unmet clinical needs of the world and China, the Company is committed to becoming an international leading enterprise in the field of innovation. Significant progress has been made in the field of biotechnological drugs, including ADCs, monoclonal antibodies, bispecific antibodies, and sought-after technologies for innovative small molecule drugs. OptiDC, an internationally renowned ADC research and development platform, has been successfully constructed by Kelun-Biotech, and 4 ADCs are in clinical trials (including two in registrational Phase III or NDA filing stage), along with multiple projects in preclinical development. At present, the company has 33 innovative projects for the treatment of serious diseases such as solid tumors, autoimmune, inflammatory, and metabolic disease, and 14 programs are advancing in clinical studies, including multiple global multicenter clinical trials which are being conducted simultaneously in several countries and regions including China, Europe, and the United States. To learn more, visit
References:
[1] Cheng Yezhe, et al. Preclinical profiles of SKB264, a novel anti-TROP2 antibody conjugated to topoisomerase inhibitor, demonstrated promising antitumor efficacy compared to IMMU-132. Frontiers in Oncology, Vol 12, 2022
Forward-Looking Statements
This press release contains certain forward-looking statements. These statements are based on the beliefs of our management as well as assumptions made by and information currently available to our management. When used in this press release, the words “aim,” “anticipate,” “believe,” “could,” “estimate,” “expect,” “going forward,” “intend,” “may,” “might,” “ought to,” “plan,” “potential,” “predict,” “project,”“seek,” “should,” “will,” “would” and the negative of these words and other similar expressions are intended to identify forward-looking statements. Such statements reflect the current views of our management with respect to future events, operations, liquidity and capital resources, some of which may not materialize or may change.
You are cautioned not to place any undue reliance on any forward-looking statements contained herein. The Company can give no assurance that these forward-looking statements will prove to have been correct. Expectations reflected in these forward-looking statements are subject to change and the Company undertakes no obligation to update or revise any forward-looking statements herein.
2023-07-11
Sichuan Kelun-Biotech Biopharmaceutical Co., Ltd. (“Kelun-Biotech”) announced its official listing on the main board of Hong Kong Exchanges and Clearing Limit...
More
Hong Kong, China, July 11, 2023 - Sichuan Kelun-Biotech Biopharmaceutical Co., Ltd. (“Kelun-Biotech”) announced its official listing on the main board of Hong Kong Exchanges and Clearing Limited (HKEX), with the stock code: 6990.HK. Shares started trading at 60.60 Hong Kong dollars (“HKD”) in the morning. By midday close, Kelun-Biotech’s share price went up by 3.80%, with a total market value of HKD13.58 billion.

As the largest Initial Public Offering (IPO) in the medical and health sector of the Hong Kong stock market in the past two years and one of the most popular investment projects in the medical and health sector recently, Kelun-Biotech’s listing on HKEX has attracted high attention and great interest from both local and international investors.
As a global unicorn enterprise, Kelun-Biotech has established a strategic partnership with Merck Sharp & Dohme LLC (together with its affiliates, “MSD”), one of the top ten multinational pharmaceutical companies in the world, and has entered into nine out-license agreements with MSD to develop up to nine ADC assets with upfront and milestone payments totaling up to USD11.8 billion. In addition, Kelun-Biotech has also received support from blue chip healthcare investors such as IDG Capital, CMG-SDIC Capital, Lilly Asia Ventures, Hillhouse, Cinda, and Sherpa, and has introduced five cornerstone investors including RTW Funds, Laurion Capital Master Fund, TruMed, CUAM, and Kelun International in this IPO.
Mr. Liu Gexin, Chairman of the Board of Kelun-Biotech said: “As the core subsidiary carrying the innovation mission of Kelun Pharmaceutical, Kelun-Biotech’s successful entry into the international capital market today marks a significant stage victory for Kelun Pharmaceutical’s ‘innovation-driven’ development strategy. With this listing as an opportunity, Kelun-Biotech will continuously improve its corporate governance structure and undertake corporate social responsibilities; accelerate the clinical research of indication-oriented anti-tumor drugs, and expand differentiated non-oncology drug portfolio; focus on key areas, and build more competitive platforms and pipelines. Leveraging Kelun Pharmaceutical’s decades-long experience in the domestic market, and the continuously deepening strategic cooperation with MSD, we will promote the development and commercialization process of planned projects, and strive to become an innovation-oriented pharmaceutical company with a global vision and industry competitiveness, to reward investors and society with excellent performance!"
Since its incorporation in 2016, Kelun-Biotech has been dedicated to the R&D, manufacturing and commercialization of novel drugs in oncology, immunology and other therapeutic areas. Kelun-Biotech is one of the first movers in the development of antibody drug conjugates (ADCs), with over a decade of accumulated experience in ADC development and is one of the first biopharmaceutical companies in China, and one of the few globally, to establish an in-house developed ADC platform, OptiDC. Relying on the platform, Kelun-Biotech has built a strong asset portfolio with 33 highly differentiated and clinically valuable pipelines centered around two ADCs, SKB264 and A166. Among the pipelines, 14 are in clinical stage, five in pivotal trial- or NDA registration-stage, nine in phase 1-or phase 2-stage.

With the Gong ringing and stock market opening at 9:30, the shares of Kelun-Biotech began to trade officially, and received a warm market response. Looking forward, Dr. Ge Junyou, Executive Director and General Manager of Kelun-Biotech said: "We will continue to advance and enhance our drug R&D capabilities. In addition to optimizing our R&D platforms and developing novel technologies to support the R&D of innovative drugs, we will strengthen the development of clinical-stage drugs in partnership with strategic partners such as MSD, in the fields of rapid indication expansion, monotherapy and combination therapy, late- and early-line. Furthermore, we will continue to expand cGMP manufacturing and quality control facilities, enhance commercialization system and capabilities, and promote the marketing of more excellent innovative drugs, to bring better treatment options for patients in China and around the world."
About Sichuan Kelun-Biotech Biopharmaceutical Co., Ltd.
Sichuan Kelun-Biotech Biopharmaceutical Co., Ltd. (“Kelun-Biotech”) is a holding subsidiary of Sichuan Kelun Pharmaceutical Co., Ltd, which focuses on the R&D, manufacture, commercialization and international cooperation of biotechnology drugs and innovative small molecule drugs. The company focuses on serious diseases such as solid tumors, autoimmune, inflammatory, and metabolic disease. Building an international drug R&D and industrialization platform to address the unmet clinical needs of the world and China, the Company is committed to becoming an international leading enterprise in the field of innovation. Significant progress has been made in the field of biotechnological drugs, including ADCs, monoclonal antibodies, bispecific antibodies, and sought-after technologies for innovative small molecule drugs. OptiDC, an internationally renowned ADC research and development platform, has been successfully constructed by Kelun-Biotech, and 4 ADCs are in clinical trials (including two in registrational Phase III or NDA filing stage), along with multiple projects in preclinical development. At present, the company has 33 innovative projects for the treatment of serious diseases such as solid tumors, autoimmune, inflammatory, and metabolic disease, and 14 programs are advancing in clinical studies, including multiple global multicenter clinical trials which are being conducted simultaneously in several countries and regions including China, Europe, and the United States. To learn more, visit http://kelun-biotech.com/.
2023-06-16
CTA of Kelun-Biotech's TROP2-ADC (SKB264, MK-2870) in combination with pembrolizumab is approved in the European Union.
More
On June 19, 2023, Kelun-Biotech announced that the innovative TROP2-ADC (SKB264, also known as MK-2870), which is being developed in collaboration with MSD (the tradename of Merck & Co., Inc, Rahway, NJ, USA), in combination with pembrolizumab (KEYTRUDA?, MSD’s anti-PD-1 therapy) for Phase II clinical studies in select patients with a variety of advanced solid tumors, has been approved for clinical trials in the European Union. This is an international multicenter clinical study which is currently approved in multiple countries and territories, including China, the United States, Canada, Australia, and now the European Union, fully demonstrating the strength of Kelun-Biotech in conducting clinical studies on a global scale. MSD and Kelun-Biotech are working closely to continually advance the international development of SKB264.
At present, immune checkpoint inhibitors, mainly represented by anti-PD-1/PD-L1 therapies, have played an important role in treating cancer across a variety of tumors. In order to improve outcomes for patients, combination therapies are being explored, including combination with chemotherapy, targeted therapy, radiotherapy, other types of immunotherapy, and more.
ADCs specifically target tumor cells to deliver cytotoxic molecules that induce immunogenic cell death (ICD) and trigger innate and adaptive immune responses, allowing large numbers of T cells to infiltrate tumor cells and thereby improving immunotherapeutic efficacy. Clinical trial data in multiple cancer types have shown that anti-PD-1 therapies in combination with ADCs may be able to improve efficacy compared to either agent alone. TROP2 is highly expressed in a variety of epithelial-derived tumors and can promote tumor cell proliferation, invasion, and metastasis, and is a promising target for broad-spectrum anti-tumor therapy. TROP2-ADCs specifically target TROP2-expressing tumor cells to deliver cytotoxic effects, and have shown encouraging anti-tumor activity as monotherapy in several clinical trials, and has the potential to improve anti-tumor efficacy in combination with immune checkpoint inhibitors such as anti-PD-1/PD-L1 therapies.
About SKB264 (MK-2870)
SKB264 is an innovative ADC targeting TROP2 which was developed by OptiDC, a well-known international ADC R&D platform of Kelun-Biotech, using a proprietary payload-linker strategy (Kthiol design strategy) that achieves an optimized balance of ADC safety and efficacy by combining novel irreversible antibody conjugation chemistry, pH-sensitive payload release mechanisms, and site-specific moderately potent toxin molecules with DAR of 7.4 (novel topoisomerase I inhibitors) [2].
SKB264 (MK-2870) has received 2 Breakthrough Therapy Designations (BTDs) from the Center for Drug Evaluation (CDE) of the National Medical Products Administration (NMPA) for the treatment of locally advanced or metastatic triple-negative breast cancer and locally advanced or metastatic EGFR-mutated non-small cell lung cancer who have failed EGFR-TKI therapy.
SKB264 (also known as MK-2870) is currently conducting Phase II and Phase III clinical trials of monotherapy/combination in multiple solid tumors. In China, the Phase III registrational clinical study of SKB264 monotherapy for patients with advanced or metastatic triple-negative breast cancer (TNBC) who have failed at least second-line therapy is progressing smoothly, and it is expected to become the first domestic TROP2-ADC approved for the Chinese market. Phase III clinical studies of SKB264 monotherapy in patients with TKI-resistant and EGFR-mutated non-small cell lung cancer (NSCLC) are also rapidly advancing. Several Phase II clinical studies of SKB264 in combination with pembrolizumab (KEYTRUDA?, MSD’s anti-PD-1 therapy) or KL-A167 (Kelun’s anti-PD-L1 monoclonal antibody) are ongoing. Kelun-Biotech has licensed the exclusive rights to MSD (the tradename of Merck & Co., Inc, Rahway, NJ, USA) to research, develop, produce and commercialize SKB264 in all territories outside of Greater China (includes Mainland China, Hong Kong, Macao, and Taiwan).
About Kelun-Biotech
Kelun-Biotech is a holding subsidiary of Sichuan Kelun Pharmaceutical Co., Ltd, which focuses on the R&D, manufacture, commercialization and international cooperation of biotechnology drugs and innovative small molecule drugs. The company focuses on serious diseases such as solid tumors, autoimmune, inflammatory, and metabolic disease. Building an international drug R&D and industrialization platform to address the unmet clinical needs of the world and China, the Company is committed to becoming an international leading enterprise in the field of innovation. Significant progress has been made in the field of biotechnological drugs, including ADCs, monoclonal antibodies, bispecific antibodies, and sought-after technologies for innovative small molecule drugs. OptiDC, an internationally renowned ADC research and development platform, has been successfully constructed by Kelun-Biotech, and 4 ADCs are in clinical trials (including two in registrational Phase III or NDA filing stage), along with multiple projects in preclinical development. At present, the company has 33 innovative projects for the treatment of serious diseases such as solid tumors, autoimmune, inflammatory, and metabolic disease, and 14 programs are advancing in clinical studies, including multiple global multicenter clinical trials which are being conducted simultaneously in several countries including China, Europe, and the United States.
References:
[1] Merck & Co., Inc. Investor Event at ASCO 2023
[2] Cheng Yezhe, et al. Preclinical profiles of SKB264, a novel anti-TROP2 antibody conjugated to topoisomerase inhibitor, demonstrated promising antitumor efficacy compared to IMMU-132. Frontiers in Oncology. Vol 12, 2022.
2023-05-27
More
2023 ASCO | Kelun Biotech SKB264 (TROP2-ADC) releases research data of non-small cell lung cancer for the first time
On May 25, US Eastern Time, the official website of the 2023 ASCO Annual Meeting published abstracts of some of the selected studies for the meeting. The innovative TROP2-ADC (SKB264, MK-2870) jointly developed by Kelun-Biotech and MSD (the tradename of Merck & Co., Inc Rahway NJ USA), with the Phase 2 expansion study data for previously treated patients with locally advanced or metastatic non-small cell lung cancer (NSCLC), has been published on the official website of ASCO.
The data cut-off is February 9, 2023, and the median follow-up time is 11.5 months.
Efficacy: 39 NSCLC patients were evaluable for efficacy (who had received SKB264 at 5 mg/kg, Q2W): the objective response rate (ORR) was 44% (17/39), the disease control rate (DCR) was 95% (37/39), the median duration of response (DoR) was 9.3 months, and the 6-month DoR rate was 77%.
In the EGFR mutant subgroup, all patients were EGFR-TKI resistant, and 50% of the patients had received at least one chemotherapy regimen. Subgroup analysis showed that the ORR was 60% (12/20), the disease control rate (DCR) was 100% (20/20), and the median progression-free survival (PFS) was 11.1 months, with 9-month PFS rate of 66.7%.
In the EGFR wild-type subgroup, all patients had previously failed anti-PD-1/L1 therapy and the median prior lines of therapy was 2. Among this subgroup, the ORR was 26% (5/19), the DCR was 89% (17/19), the median PFS was 5.3 months, and the 9-month overall survival (OS) rate was 80.4%.
Safety: Grade ≥3 treatment-related adverse events (TRAEs) were reported in 67.4% (29/43) of patients. The most common Grade ≥3 TRAEs (≥ 5%) were decreased neutrophil count, anemia, decreased white blood cell count, oral mucositis, rash, and decreased lymphocyte count. Most blood-related adverse events occurred within two months of starting SKB264 treatment and recovered after treatment with granulocyte colony-stimulating factor and erythropoietin. TRAEs led to a dose reduction rate of 23.3%, and no discontinuation or death due to TRAEs occurred. No neurotoxicity or drug-related interstitial lung disease (ILD)/pneumonitis was observed.
In previously treated patients with locally advanced or metastatic NSCLC, SKB264 demonstrated encouraging antitumor activity and a manageable safety profile. A Phase 3 registrational study of SKB264 for the treatment of locally advanced or metastatic NSCLC is in progress.
About SKB264 (TROP2-ADC)
SKB264 is a new generation of antibody-drug conjugates (ADC) composed of a humanized
monoclonal antibody targeting TROP2, an enzymatically cleavable linker and a novel topoisomerase I inhibitor for which Kelun-Biotech has independent intellectual property rights. SKB264 combines the specificity of monoclonal antibodies to target antigens on the surface of tumor cells and the high cell-killing efficiency of cytotoxic drugs. SKB264 has been granted breakthrough therapy designation by the Center for Drug Evaluation (CDE) of the National Medical Products Administration (NMPA) for the treatment of locally advanced or metastatic triple-negative breast cancer and locally advanced or metastatic EGFR-mutated non-small cell lung cancer.
Based on preliminary clinical data, SKB264 is currently conducting Phase 2 and Phase 3 clinical trials involving monotherapy and combination therapy for multiple tumor types. In China, the Phase 3 registrational clinical study of SKB264 monotherapy for advanced or metastatic TNBC patients who have failed at least second-line treatment is progressing smoothly, and it is expected to become the first domestic TROP2-ADC approved for marketing. Phase 3 clinical research of SKB264 monotherapy for TKI-resistant EGFR-mutated non-small cell lung cancer patients is also rapidly advancing. Several Phase 2 clinical studies of SKB264 combined with pembrolizumab (KEYTRUDA?, MSD’s anti-PD-1 therapy) or KL-A167 (anti-PD-L1 monoclonal antibody) are under development. In addition, Kelun-Biotech has granted an exclusive license to MSD to manufacture, develop, and commercialize SKB264 (also known as MK-2870) outside of Greater China territories (Greater China includes Mainland China, Hong Kong, Macau and Taiwan). Global clinical research in several major countries, including United States, Canada, Australia, France, Spain, Belgium and Poland, are currently ongoing while additional multi-center clinical studies are also under preparation.
About Kelun-Biotech
Kelun-Biotech is a holding subsidiary of Kelun Pharmaceutical, focusing on the R&D, production, commercialization and international cooperation of innovative biologics and small molecule drugs. Focusing on the unmet clinical needs in China and the rest of world, the company aims to treat major diseases, including solid tumors, autoimmune, inflammatory and metabolic diseases. By building an international drug R&D and industrialization platform, the company strives to become an international leading enterprise in the field of innovation. Significant progress has been made in the field of biologic drugs, including ADC, monoclonal antibody, bispecific antibody, as well as novel targets for innovative small molecule drug discovery, etc. The world-renowned ADC R&D platform, OptiDC, has been successfully established, from which 4 ADCs are in the clinical research stage, and several are in the preclinical research stage. The company currently has 33 innovative projects for the treatment of major diseases such as solid tumors, autoimmune, inflammatory, and metabolic disease, including 14 projects advancing in clinical research, of which 3 are being carried out concurrently in China and the United States.
Business Development Team
Saturday, May 27, 2023
2023-05-27
More
KLUS Pharma will attend 2023 ASCO Annual Meeting in Chicago from June 2-6th.
KLUS Pharma is the US subsidiary of Kelun-Biotech, a mid-sized innovative biopharma company based in Chengdu, China. Kelun-Biotech is committed to the development of innovative therapies to address the unmet needs of patients with advanced cancer and other serious illnesses. Currently, we are advancing several antibody-drug conjugates (ADCs) and immuno-oncology assets in ongoing clinical trials for advanced solid tumors, including lung, breast, gastric, and others. We are excited to share recent progress and review the latest data updates being presented at ASCO and look forward to meeting potential collaborators and colleagues!
Business Development Team
Saturday, May 27, 2023
2022年09月06日
我们决定向甘孜泸定、雅安石棉捐赠300万元现金、300万元物资,目前已成功对接甘孜州红十字会、雅安市红十字会,今天下午已经完成打款,物资根据当地所需正在紧急集结。对于灾区需要的其他支持,我们也当全力以赴。”
9月6日下午,四川科伦药业股份有限公司相关负责人告诉记者,针对四川泸定6.8级地震中受灾严重的泸定县和石棉县,他们紧急启动灾害应急处理方案,并进行现金和物资捐赠。